Organic Nitrogen Fertilizers for Container Substrates
by Richard Evans
It has been half a century since organic fertilizers were widely used as sources of nitrogen in container mixes, but there has been a resurgence of interest in them in recent years. In large part, the new interest is the result of the niche market created by a demand for “green,” organically-grown plants, but organic nitrogen fertilizers have another attractive feature as well. Organic nitrogen is insoluble, so it serves as a storage form that reduces the likelihood of nitrogen losses from the pot due to leaching and runoff.
The nitrogen stored in organic fertilizers becomes available when chemical or microorganism activity converts it to soluble forms through a process called mineralization. The first breakdown product is ammonium. Microorganisms can also convert ammonium to nitrate, which is the normal end product of mineralization. The conversion process is affected by several factors, including the population of microorganisms; soil temperature, moisture content and aeration; and the type and amount of organic matter. Organic fertilizers are usually ineffective at temperatures below 40ºF, and the conversion of ammonium to nitrate is particularly sensitive to temperature.
Much of the research on mineralization of organic nitrogen in soilless mixes was done by UC researchers in the 1950s. The group responsible for the UC Manual 23, The UC System for Producing Healthy Container-Grown Plants, published useful information about the availability of nitrogen from organic fertilizers in soilless mixes (Baker 1957). For example, table 1 here presents their data describing the effect of temperature and organic fertilizer type on nitrogen mineralization. The authors concluded that blood meal or hoof and horn meal would be the best organic nitrogen fertilizer for container-grown crops because they released nitrogen more slowly, which would make them effective for a longer time.
Table 1. Nitrogen available from mineralization of organic nitrogen sources in steam pasteurized and unpasteurized soilless media. Data from Baker (1957).
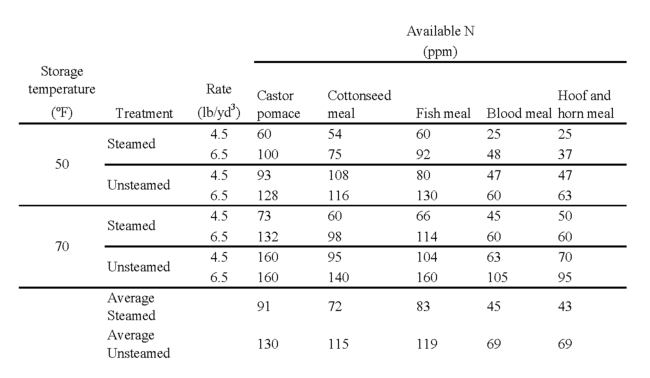
The UC Manual group tested its organic fertilizers on bedding plant crops, which it chose because the short cropping period for bedding plants meant they could get quick results. Later research showed that mineralization of organic nitrogen sources ends too soon to sustain the growth of most container-grown plants. For example, Williams and Nelson (1992) tested several organic nitrogen sources, including sewage sludge, poultry manure sludge, bonemeal, pine needles, and poultry feathers, and found that all ceased releasing sufficient nitrogen after six to seven weeks. Hartz and Johnstone (2006) reported that the nitrogen in seabird guano, fish powder, feather meal and blood meal was mineralized within six to eight weeks. These results, and others like them, indicate that the common organic fertilizers cannot sustain most container-grown crops unless they are supplemented with liquid fertilizers or subsequent top-dressing with solid fertilizers.
The organic nitrogen sources discussed above are waste products. We recently tested a promising new source of organic nitrogen that isn’t technically a waste product, but is treated like one: dried jellyfish. Jellyfish populations have expanded rapidly over the past couple of decades, probably because climate change has warmed oceans and water pollution and overfishing have removed their predators and competitors. Exploding populations of jellyfish are wreaking havoc with commercial fisheries and, surprisingly, nuclear power generators. For example, a few years ago, the USS Ronald Reagan, the world’s most advanced aircraft carrier at the time, was completely disabled because it sucked thousands of jellyfish into the cooling system of its nuclear engine. Recently several nuclear power plants that are cooled with ocean water have been in the news because of shutdowns caused by jellyfish clogging the cooling water intake lines. Some power plants must remove as much as 150 tons of jellyfish each day. It is costly to deal with the jellyfish, but it is possible that some of the cost could be recouped if dried jellyfish could be sold as fertilizer. It seems to hold promise, since dried jellyfish contains 14% nitrogen by weight.
We conducted a greenhouse experiment to test whether the mineralization rate of organic nitrogen in the dried jellyfish is sufficient to meet the nitrogen demand of ‘Golden Gate’ chrysanthemum grown in a soilless mix. We incorporated either dried jellyfish or a conventional controlled-release fertilizer into the mix at a rate of 1.9 grams of N per 6-inch pot.
No treatment differences were apparent during the first six weeks. Leaf color (assessed with a SPAD chlorophyll meter) and growth rates were normal for both treatments. After seven weeks, however, we noticed slight yellowing of older leaves and a slower growth rate of plants fertilized with jellyfish — both signs of incipient nitrogen deficiency. At the end of the experiment the dry weight of plants fertilized with jellyfish was lower (37.2 grams) than the dry weight of plants fertilized with controlled release fertilizer (44.6 grams). Therefore, like organic fertilizers made from waste products, the jellyfish fertilizer was able to meet the nitrogen demand of chrysanthemum for the first several weeks after planting, but was not sufficient to satisfy the nitrogen requirement for the whole cropping period.
Thus far, efforts to extend the release period of organic nitrogen fertilizers have been unsuccessful. For example, plants produced in a potting mix containing a pelletized organic nitrogen source did not grow as well as those that received a conventional fertilizer (Mikkelsen 2003). For now, at least, organic production of container plants must depend on supplemental applications of fertilizer after planting.
Richard Evans is UC Cooperative Extension Environmental Horticulturist, Department of Plant Sciences, UC Davis.
References
Baker KF (editor). 1957. The UC System for Producing Healthy Container-grown Plants through the Use of Clean Soil, Clean Stock, and Sanitation. University of California, Division of Agricultural Sciences, Agricultural Experiment Station, Extension Service Manual 23.
Hartz TK, Johnstone PR. 2006. Nitrogen availability from high-nitrogen-containing organic fertilizers. HortTechnology 16:39-42.
Mikkelsen RL. 2003. Using tobacco by-products as a nitrogen source for container-grown houseplants. Journal of Plant Nutrition 26:1697-1708.
Williams KA, Nelson PV. 1992. Low, controlled nutrient availability provided by organic waste materials for Chrysanthemum. Journal of the American Society for Horticultural Science 117:422-429.












