- Author: Surendra K. Dara
Strawberry, a high-value specialty crop in California, suffers from several soilborne, fruit, and foliar diseases. Verticillium wilt caused by Verticillium dahliae, Fusarium wilt caused by Fusarium oxysporum f. sp. fragariae, and Macrophomina crown rot or charcoal rot caused by Macrophomina phaseolina are major soilborne diseases that cause significant losses without proper control. Chemical fumigation, crop rotation with broccoli, nutrient and irrigation management to minimize plant stress, and non-chemical soil disinfestation are usual control strategies for these diseases. Botrytis fruit rot or gray mold caused by Botrytis cineaea is a common fruit disease requiring frequent fungicidal applications. Propagules of gray mold fungus survive in the soil and infect flowers and fruits. A study was conducted to evaluate the impact of drip application of various fungicides on improving strawberry health and enhancing fruit yields.
Methodology
This study was conducted in an experimental strawberry field at the Shafter Research Station during 2019-2020. Cultivar San Andreas was planted on 28 October 2019. No pre-plant fertilizer application was made in this non-fumigated field which had Fusarium wilt, Macrophomina crown rot, and Botrytis fruit rot in the previous year's strawberry planting. Each treatment was applied to a 300' long bed with single drip tape in the center and two rows of strawberry plants. Sprinkler irrigation was provided immediately after planting along with drip irrigation, which was provided one or more times weekly as needed for the rest of the experimental period. Each bed was divided into six 30' long plots, representing replications, with an 18' buffer in between. Between 6 November 2019 and 9 May 2020, 1.88 qt of 20-10-0 (a combination of 32-0-0 urea ammonium nitrate and 10-34-0 ammonium phosphate) and 1.32 qt of potassium thiosulfate was applied 20 times at weekly intervals through fertigation. Treatments were applied either as a transplant dip or through the drip system using a Dosatron fertilizer injector (model D14MZ2). The following treatments were evaluated in this study:
i) Untreated control: Neither transplants nor the planted crop was treated with any fungicides.
ii) Abound transplant dip: Transplants were dipped in 7 fl oz of Abound (azoxystrobin) fungicide in 100 gal of water for 4 min immediately prior to planting. Transplant dip in a fungicide is practiced by several growers to protect the crop from fungal diseases.
iii) Rhyme: Applied Rhyme (flutriafol) at 7 fl oz/ac immediately after and 30, 60, and 90 days after planting through the drip system.
iv) Velum Prime with Switch: Applied Velum Prime (fluopyram) at 6.5 fl oz/ac 14 and 28 days after planting followed by Switch 62.5 WG (cyprodinil + fludioxinil) at 14 oz/ac 42 days after planting through the drip system.
v) Rhyme with Switch: Four applications of Rhyme at 7 fl oz/ac were made 14, 28, 56, and 70 days after planting with a single application of Switch 62.5 WG 42 days after planting through the drip system.
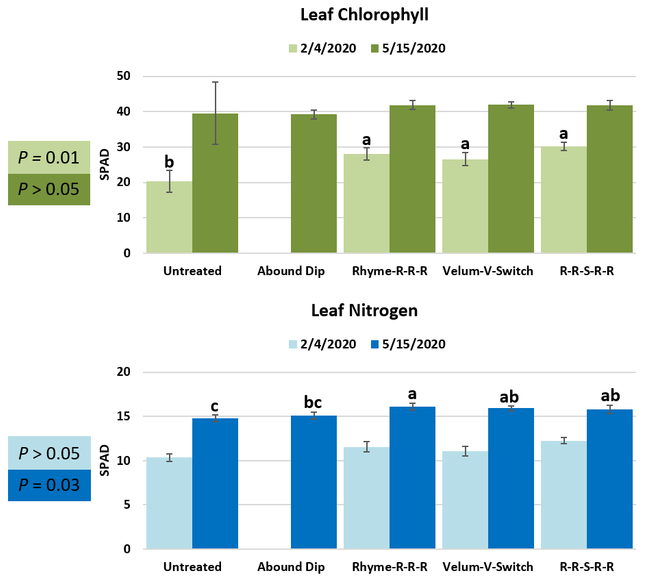
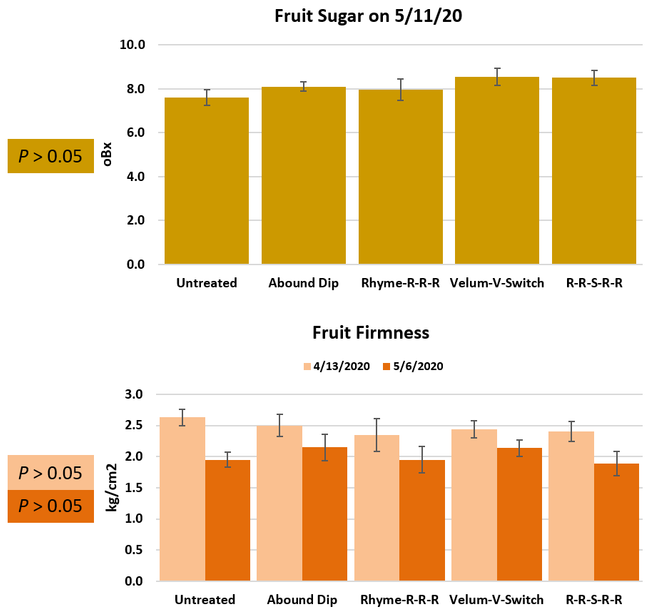
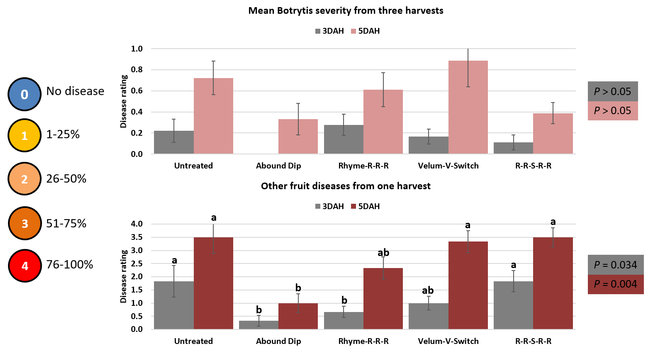
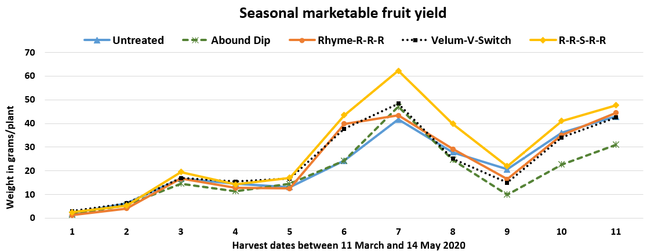
Parameters observed during the study included leaf chlorophyll and leaf nitrogen (with chlorophyll meter) in February and May; fruit sugar (with refractometer) in May; fruit firmness (with penetrometer) in April and May; severity of gray mold (caused by Botrytis cinereae) twice in March and once in May, and other fruit diseases (mucor fruit rot caused by Mucor spp. and Rhizopus fruit rot caused by Rhizopus spp.) once in May 3 and 5 days after harvest (on a scale of 0 to 4 where 0=no infection; 1=1-25%, 2=26-50%, 3=51-75% and 4=76-100% fungal growth); and fruit yield per plant from 11 weekly harvests between 11 March and 14 May 2020. Leaf chlorophyll and nitrogen data for the Abound dip treatment were not collected in February. Data were analyzed using analysis of variance in Statistix software and significant means were separated using the Least Significant Difference test.
Results and Discussion
Leaf chlorophyll content was significantly higher in plants that received drip application of fungicides compared to untreated plants in February while leaf nitrogen content was significantly higher in the same treatments during the May observation. There were no differences in fruit sugar or average fruit firmness among the treatments.
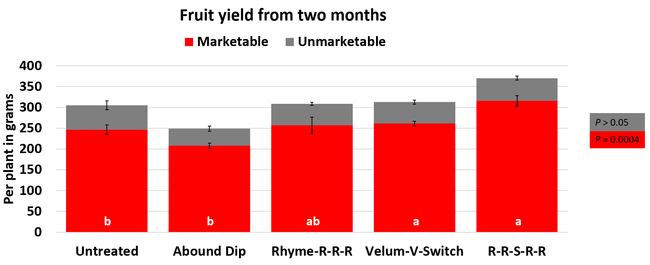
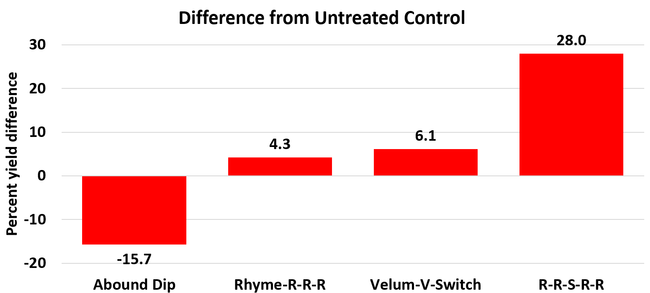
The average gray mold severity from three harvest dates was low and did not statistically differ among the treatments. However, the severity of other diseases was significantly different among various treatments with the lowest rating in Abound transplant dip on both 3 and 5 days after harvest and only 3 days after harvest in plants that received four applications of Rhyme. Unlike the previous year, visible symptoms of the soilborne diseases were not seen during the study period to evaluate the impact of the treatments. However, there were significant differences among treatments for the marketable fruit yield. The highest marketable yield was observed in the treatment that received Rhyme and Switch followed by Velum Prime and Switch and Rhyme alone. The lowest fruit yield was observed in Abound dip treatment. Unmarketable fruit (deformed or diseased) yield was similar among the treatments. Compared to the untreated control, Abound dip resulted in 16% less marketable yield and such a negative impact from transplant dip in fungicides has been seen in other studies (Dara and Peck, 2017 and 2018; Dara, 2020). Marketable fruit yield was 4-28% higher where fungicides were applied to the soil.
Although visible symptoms of soilborne diseases were absent during the study, periodic drip application of the fungicides probably suppressed the fungal inocula and associated stress and might have contributed to increased yields. The direct impact of fungicide treatments on soilborne pathogens was, however, not clear in this study due to the lack of disease symptoms. Considering the cost of chemical fumigation or soil disinfestation and the environmental impact of chemical fumigation, treating the soil with fungicides can be an economical option if they are effective. While this study presents some preliminary data, additional studies in non-fumigated fields in the presence of pathogens are necessary to consider soil fungicide treatment as a control option.
Acknowledgments: Thanks to FMC for funding this study and Marjan Heidarian Dehkordi and Tamas Zold for their technical assistance.
References
Dara, S. K. 2020. Improving strawberry yields with biostimulants and nutrient supplements: a 2019-2020 study. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=43631
Dara, S. K. and D. Peck. 2017. Evaluating beneficial microbe-based products for their impact on strawberry plant growth, health, and fruit yield. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=25122
Dara, S. K. and D. Peck. 2018. Evaluation of additive, soil amendment, and biostimulant products in Santa Maria strawberry. CAPCA Adviser, 21 (5): 44-50.
- Author: Surendra K. Dara
Botrytis fruit rot or gray mold, caused by Botrytis cinerea, is common fruit disease in California strawberries (Koike et al. 2018). Botrytis cinerea has a wide host range infecting several commercially important crops including blueberry (Saito et al. 2016), grapes (Saito et al., 2019), and tomato (Breeze, 2019). Fungal infection can cause flower or fruit rot. Fruit can be infected directly or through a latent infection in the flowers. Moist and cool conditions favor fungal infections and increased sugar content in the ripening fruit can also contribute to the disease development. Initial symptoms of infection appear as brown lesions and a thick mat of gray conidia is characteristic symptom in the later stages of infection. As chemical fungicides are primarily used for gray mold control, fungicide resistance is a common problem around the world (Panebianco et al., 2015; Liu et al., 2016; Stockwell et al., 2018; Weber and Hahn, 2019). In strawberry, cultural control options such as removing diseased plant material or using cultivars with traits that can reduce gray mold infections may not be practical when the disease is widespread in the field or cultivar choice is made based on other factors. Non-chemical control options are necessary to help reduce the risk of chemical fungicide resistance, prolong the life of available chemical fungicides, achieve desired disease control, and to maintain environmental health. Although there are several botanical and microbial fungicides available for gray mold control, limited information is available on their efficacy in California strawberries. A study was conducted in the spring of 2019 to evaluate the efficacy of several chemical, botanical, and microbial fungicides in certain combinations and rotations to help identify effective options for an integrated disease management strategy.
Methodology
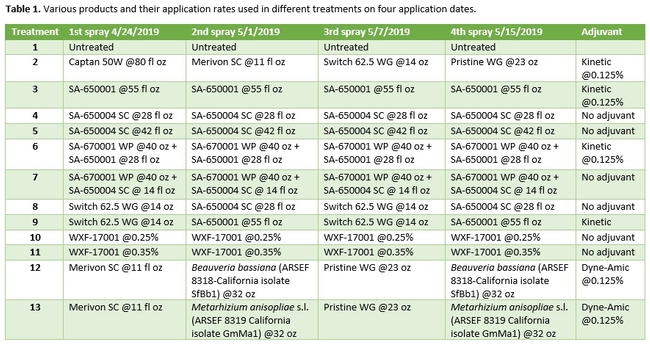
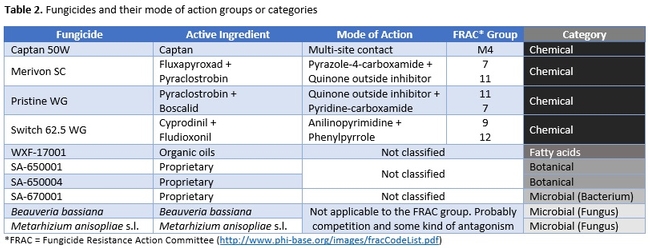
Strawberry cultivar San Andreas was planted late November, 2018 and the study was conducted in April and May, 2019. Each treatment had a 20' long strawberry plot with two rows of plants replicated in a randomized complete block design. Plots were maintained without any fungicidal applications until the study was initiated. Table 1 contains the list of treatments, application rates and dates of application, and Table 2 contains the type of fungicide used and their mode of action. Beauveria bassiana and Metarhizium anisopliae s.l. are California isolates of entomopathogenic fungi, isolated from an insect and a soil sample, respectively. These fungi are pathogenic to a variety of arthropods and some strains are formulated as biopesticides for arthropod control. However, earlier studies in California demonstrated that these fungi are also known to antagonize plant pathogens such as Fusarium oxysporum f.sp. vasinfectum Race 4 (Dara et al., 2016) and Macrophomina phaseolina (Dara et al., 2018) and reduce the disease severity. To further evaluate their efficacy against B. cinerea, these two fungi were also included in this study alternating with two chemical fungicides.
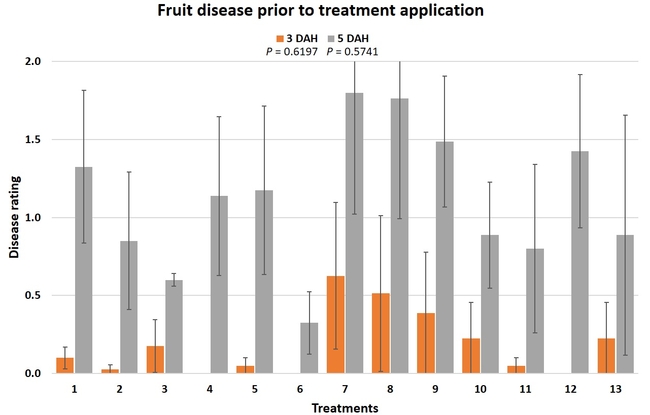
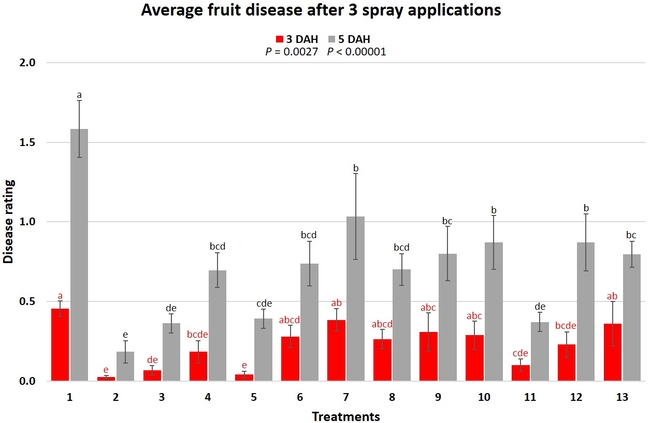
Treatments were applied with a CO2-pressurized backpack sprayer using 66.5 gpa spray volume. Five days before the first spray application and 3 days after each application, all ripe fruit were harvested from each plot and incubated at the room temperature in vented plastic containers. The level of gray mold on fruit from each plot was rated using a 0 to 4 scale (where 0=no disease, 1=1-25% fruit with fungal infection, 2=26-50% infection, 3=51-75%, and 4=76-100%) 3 and 5 days after each harvest (DAH). Due to the rains, fruit could not be harvested after the 3rd spray application for disease rating, but was harvested and discarded after the rains to avoid cross infection for the following week's harvest. Data were analyzed using analysis of variance using Statistix software and significant means were separated using Least Significant Difference separation test.
Results
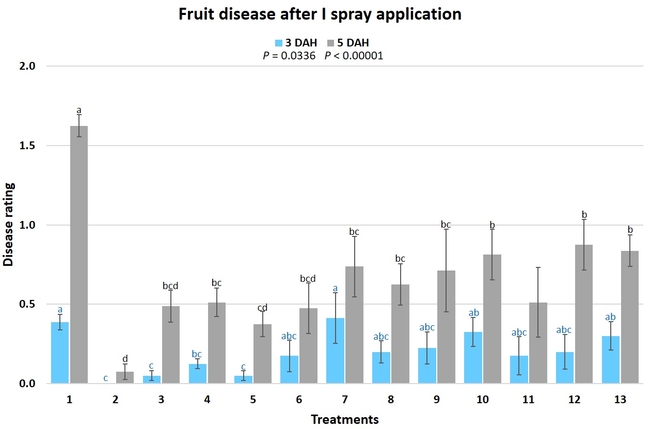
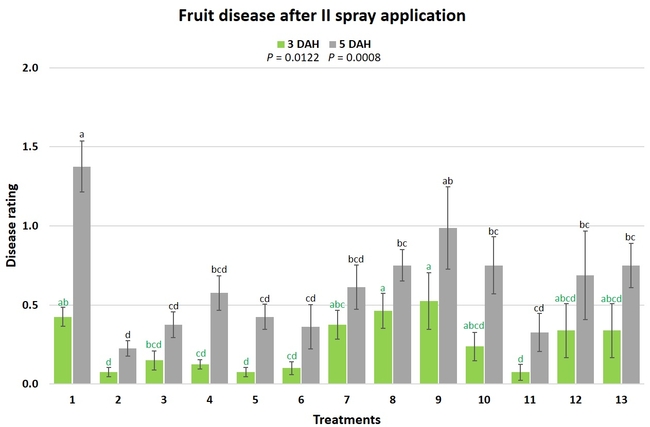
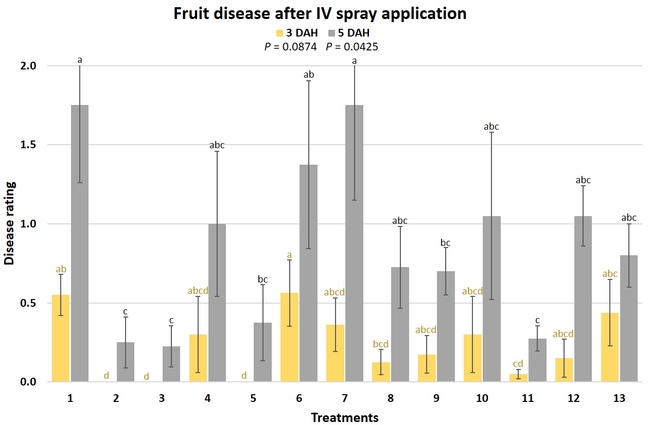
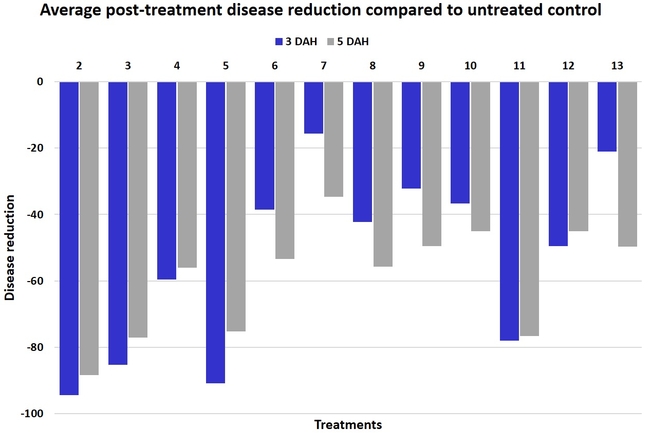
Gray mold occurred at low to moderate levels during the study period. Along with B. cinerea, there were a few instances of minor fungal infections from Rhizopus spp. (Rhizopus fruit rot) and Mucor spp. (Mucor fruit rot). Pre-treatment disease ratings were statistically not significant (P = 0.6197 and 0.5741) 3 and 5 DAH. While the chemical standard treatment with the rotation of Captan, Merivon, Switch, and Pristine (treatment 2) appeared to result in the lowest disease rating throughout the observation period, treatments 3 and 5 after the 1st spray application, treatments 5 and 11 along with 3, 4 and 6 after the 2nd spray application, and treatments 3 and 5 along with 11 after the 4th spray application also had similar disease control at 3 DAH. When disease at 5 DAH was compared, the lowest rating was seen in treatment 2 after the 1st and 2nd spray applications, and treatments 2, 3, and 11 after the 4th application. Several other treatments also provided statistically similar control during these days.
When the average disease rating for the three post-treatment observation events was considered, treatment 2, 3, 5, and 11 had the lowest disease at both 3 and 5 DAH. Treatments 4 and 12 at 3 DAH also had a statistically similar level of disease control to treatment 2.
In general, most of the treatments provided moderate to high control compared to the disease in untreated control when the post-treatment averages were considered. Only treatment 7 and 13 had lower control at 3 DAH.
Discussion
This study compared a variety of registered and developmental products along with two entomopathogenic fungi in managing B. cinerea. Considering the fungicide resistance problem in B. cinerea in multiple crops, having multiple non-chemical control options is very important to achieve desirable control with integrated disease management strategies. Since the active ingredients in the botanical and bacterial fungicides used in this study are not public, discuss will be limited on their modes of action and efficacy at this point. Similarly, the active ingredient of WXF-17001 is also not known, however, an earlier study by Calvo-Garrido et al. (2014) demonstrated that a fatty acid-based natural product reduced B. cinerea conidial germination by 54% and disease severity in grapes by 96% compared to untreated control. The product used by Calvo-Garrido et al. (2014) is thought to be fungistatic and reduce the postharvest respiratory activity and ethylene production in fruits.
While chemical fungicides have a specific mode of action, biological and other products act in multiple manners either directly antagonizing the plant pathogen or by triggering the plant defenses. For example, amending the potting medium with biochar resulted in induced systemic resistance in tomato and reduced B. cinerea severity by 50% (Mehari et al., 2015). Luna et al. (2016) also showed that application of β-aminobutyric acid and jasmonic acid promoted seed germination and long-term resistance to B. cinerea in tomato. Burkholderia phytofirmans, beneficial endophytic bacterium, offered protection against B. cinerea in grapes by mobilizing carbon resources (callose deposition), triggering plant immune system (hydrogen peroxide production and priming of defense genese), and through antifungal activity (Miotto-Vilanova et al. 2016). Similarly, entomopathogenic fungi such as B. bassiana are also known to induce systemic resistance against plant pathogens (Griffin et al. 2006). Compared to other options evaluated in the study, entomopathogenic fungi have an advantage of controlling both arthropod pests and diseases, while also having plant growth promoting effect (Dara et al. 2017).
Rotating fungicides with different mode of actions reduces the risk of resistance development and using some combinations will also maintain control efficacy. This study provided the efficacy of multiple control options and their combinations and rotations for B. cinerea. This is also the first study demonstrating the efficacy of entomopathogenic fungi against B. cinerea in strawberry.
Acknowledgements: Thanks to Sipcam Agro and Westbridge for funding the study, technical assistance of Hamza Khairi for data collection, and the field staff at the Shafter Research Station for the crop maintenance.
References
Breeze, E. 2019. 97 Shades of gray: genetic interactions of the gray mold, Botrytis cinerea, with wild and domesticated tomato. The Plant Cell 31: 280-281. https://doi.org/10.1105/tpc.19.00030
Calvo-Garrido, C., A.A.G. Elmer, F. J. Parry, I. Viñas, J. Usall, R. Torres, R.H. Agnew, and N. Teixidó. 2014. Mode of action of a fatty acid-based natural product to control Botrytis cinerea in grapes. J. Appl. Microbiol. 116: 967-979. https://doi.org/10.1111/jam.12430
Dara, S. K., S. S. Dara, S.S.R. Dara, and T. Anderson. 2016. First report of three entomopathogenic fungi offering protection against the plant pathogen, Fusarium oxysporum f.sp. vasinfectum. UC ANR eJournal of Entomology and Biologicals https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=22199
Dara, S. K., S.S.R. Dara, and S. S. Dara. 2017. Impact of entomopathogenic fungi on the growth, development, and health of cabbage growing under water stress. Amer. J. Plant Sci. 8: 1224-1233. https://doi.org/10.4236/ajps.2017.86081
Dara, S.S.R., S. S. Dara, and S. K. Dara. 2018. Preliminary report on the potential of Beauveria bassiana and Metarhizium anisopliae s.l. in antagonizing the charcoal rot causing fungus Macrophomina phaseolina in strawberry. UC ANR eJournal of Entomology and Biologicals https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=28274
Griffin, M. R., B. H. Ownley, W. E. Klingeman, and R. M. Pereira. 2006. Evidence of induced systemic resistance with Beauveria bassiana against Xanthomonas in cotton. Phytopathol. 96.
Koike, S. T., G. T. Browne, T. R. Gordon, and M. P. Bolda. 2018. UC IPM pest management guidelines: strawberry (diseases). UC ANR Publication 3468. https://www2.ipm.ucanr.edu/agriculture/strawberry/Botrytis-Fruit-Rot/
Liu, S., Z. Che, and G. Chen. 2016. Multiple-fungicide resistance to carbendazim, diethofencardb, procymidone, and pyrimethanil in field isolates of Botrytis cinerea from tomato in Henan Province, China. Crop Protection 84: 56-61.
Luna, E., E. Beardon, S. Ravnskov, J. Scholes, and J. Ton. 2016. Optimizing chemically induced resistance in tomato against Botrytis cinerea. Plant Dis. 100: 704-710. https://doi.org/10.1094/PDIS-03-15-0347-RE
Mehari, Z. H., Y. Elad, D. Rav-David, E. R. Graber, and Y. M. Harel. 2015. Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant and Soil 395: 31-44.
Miotto-Vilanova, L., C. Jacquard, B. Courteaux, L. Wortham, J. Michel, C. Clément, E. A. Barka, and L. Sanchez. 2016. Burkholderia phytofirmans PsJN confers grapevine resistance against Botrytis cinerea via a direct antimicrobial effect combined with a better resource mobilization. Front. Plant Sci. 7: 1236. https://doi.org/10.3389/fpls.2016.01236
Panebianco, A., I. Castello, G. Cirvilleri, G. Perrone, F. Epifani, M. Ferrarra, G. Polizzi, D. R. Walters, and A. Vitale. 2015. Detection of Botrytis cinerea field isolates with multiple fungicide resistance from table grape in Sicily. Crop Protection 77: 65-73.
Saito, S., T. J. Michailides, and C. L. Xiao. 2016. Fungicide resistance profiling in Botrytis cinerea populations from blueberry in California and Washington and their impact on control of gray mold. Plant Dis. 100: 2087-2093. https://doi.org/10.1094/PDIS-02-16-0229-RE
Saito, S., T. J. Michailides, and C. L. Xiao. 2019. Fungicide-resistant phenotypes in Botrytis cinerea populations and their impact on control of gray mold on stored table grapes in California. European J. Plant Pathol. 154: 203-213.
Stockwell, V. O., B. T> Shaffer, L. A. Jones, and J. W. Pscheidt. 2018. Fungicide resistance profiles of Botrytis cinerea isolated from berry crops in Oregon. Abstract for International Congress of Plant Pathology: Plant Health in A Global Economy; 2018 July 29-Aug 3; Boston, MA.
Weber, R.W.S. and M. Hahn. 2019. Grey mould disease of strawberry in northern Germany: causal agents, fungicide resistance and management strategies. Appl. Microbiol. Biotechnol. 103: 1589-1597.
- Author: Sumanth S. R. Dara
- Author: Suchitra S. Dara
- Author: Surendra K. Dara
Charcoal rot, caused by Macrophomina phaseolina, is one of the important fungal diseases of strawberry in California. Macrophomina phaseolina is a soilborne fungus and has a wide host range, including alfalfa, cabbage, corn, pepper, and potato, some of which are cultivated in the strawberry production areas in California. The fungus infects the vascular system of the plant roots, obstructing the nutrient and water supply and ultimately resulting in stunted growth, wilting, and death of the plant. The fungus survives in the soil and infected plant debris as microsclerotia (resting structures made of hyphal bodies) and can persist for up to three years. Microslerotia germinate and penetrate the root system to initiate infection. Plants are more vulnerable to fungal infection when they are experiencing environmental (extreme weather or drought conditions) and physiological (heavy fruit bearing) stress.
Soil fumigation is the primary management option for addressing charcoal rot in strawberry. Crop rotation with broccoli can also reduce the risk of charcoal rot due to glucosinolates and isothiocyanates in broccoli crop residue that have fungicidal properties. Beneficial microorganisms such as Bacillus spp. and Trichoderma spp. are also considered, especially in organic strawberries, to antagonize M. phaseolina and other soilborne pathogens and provide some protection. The role of beneficial microbes in disease management or improving crop growth and health is gaining popularity in the recent years with the commercial availability of biofungicide, biostimulant, and soil amendment products. In a couple of recent strawberry field studies in Santa Maria, some of the beneficial microbial products improved fruit yield or crop health. These treatments can be administered by inoculating the transplants prior to planting, immediately after planting or periodically applying to the plants and or the soil. Adding beneficial microbes can help improve the soil microbiome especially after chemical or bio-fumigation and anaerobic soil disinfestation.
Similar to the benefits of traditionally used bacteria (e.g., Bacillus spp. and Pseudomonas spp.) and fungi (e.g., Glomus spp. and Trichoderma spp.), studies with entomopathogenic fungi such as Beauveria bassiana, Isaria fumosorosea, and Metarhizium spp. also demonstrated their role in improving water and nutrient absorption or antagonizing plant pathogens. The advantage of entomopathogenic fungi is that they are already used for arthropod pest management in multiple crops, including strawberry; now, there are the additional benefits of promoting crop growth and antagonizing plant pathogens. In light of some promising recent studies exploring these roles, a study was conducted using potted strawberry plants to evaluate the efficacy of two California isolates of Beauveria bassiana and Metarhizium anisopliae s.l. and their application strategies against M. phaseolina.
Methodology
About 6 week old strawberry plants (cultivar Albion) from a strawberry field at the Shafter Research Station were transplanted into 1.6-gallon pots with Miracle-Gro All Purpose Garden Soil (0.09-0.05-0.07 N-P-K) and maintained in an outdoor environment. They were regularly watered, and their health was monitored for about 5 months prior to the commencement of the study. Conidial suspensions of the California isolates of B. bassiana and M. anisopliae s.l. were applied one week before, after, or at the time of applying microsclerotia of M. phaseolina to the potting mix. The following treatments were evaluated in the study:
- Untreated control
- Soil inoculated with M. phaseolina
- Soil inoculated with B. bassiana 1 week prior to M. phaseolina inoculation
- Soil inoculated with M. anisopliae s.l. 1 week prior to M. phaseolina inoculation
- Soil inoculated with B. bassiana at the time of M. phaseolina inoculation
- Soil inoculated with M. anisopliae s.l. at the time of M. phaseolina inoculation
- Soil inoculated with B. bassiana 1 week after to M. phaseolina inoculation
- Soil inoculated with M. anisopliae s.l. 1 week after to M. phaseolina inoculation
Entomopathogenic fungi were applied as 1X1010 viable conidia in 100 ml of 0.01% Dyne-Amic (surfactant) solution distributed around the plant base. To apply M. phaseolina, 5 grams of infested cornmeal-sand inoculum containing 2,500 CFU/gram was added to four 5 cm deep holes around the base of the plant. Each treatment had six pots each planted with a single strawberry plant representing a replication. Treatments were randomly arranged within each replication. The study was repeated once a few days after the initiation of the first experiment.
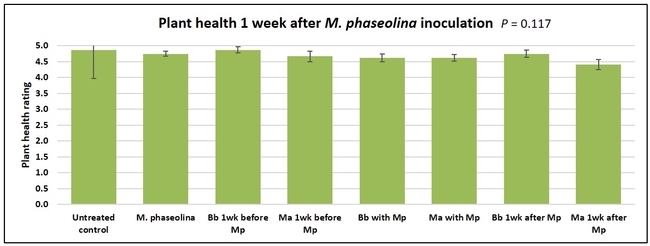
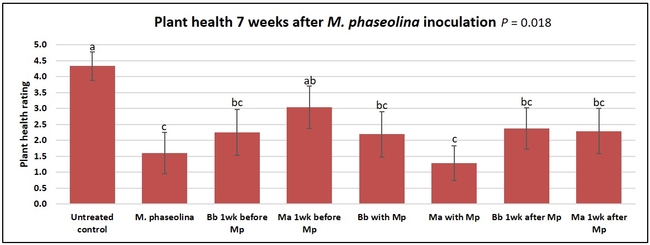
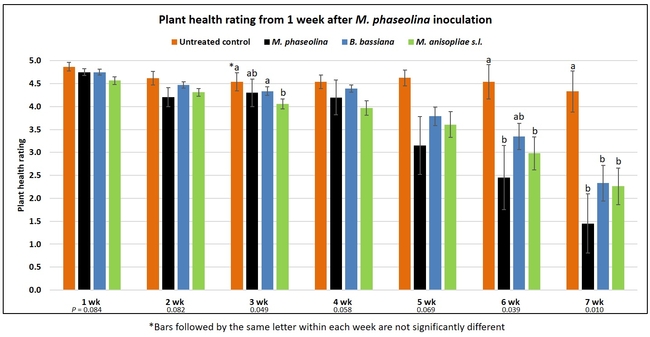
Plant health was monitored starting from the first week after the M. phaseolina inoculation and continued for seven weeks. Plant health was rated on a scale of 0 to 5 where 0=dead and 5=very healthy and the rest of the ratings in between depending on the extent of wilting. Data from both experiments were combined and analyzed by ANOVA using Statistix software and significant means were separated using LSD test. The influence of entomopathogenic fungal treatments applied at different times as well as the combined effect of different applications within each fungus were compared for seven weeks. Ratings for some plants that were scorched from hot summer temperatures and died abruptly were removed from the analyses.
Results
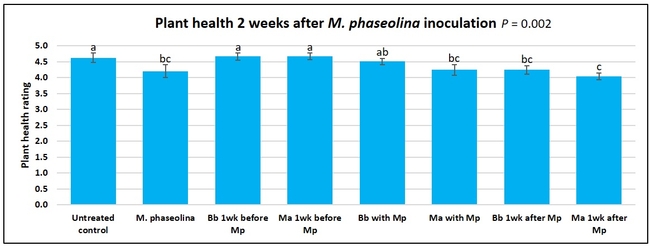
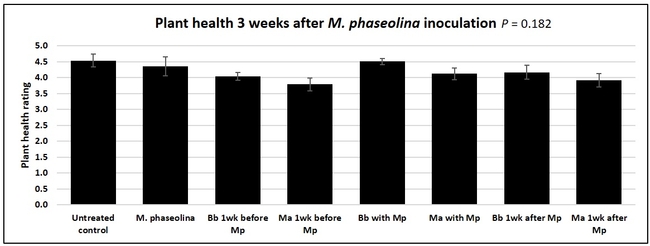
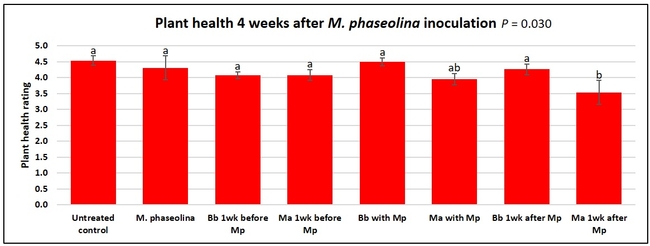
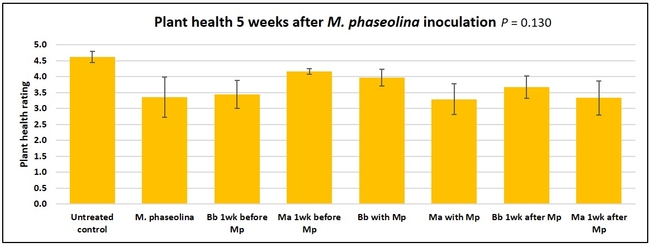
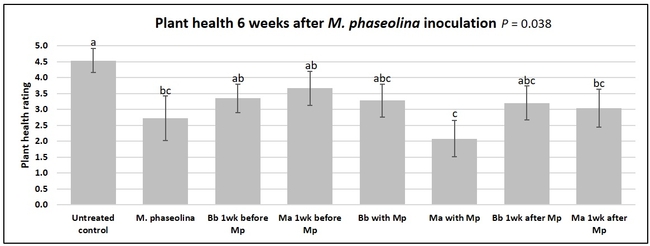
Untreated control plants maintained good health throughout the observation period varying between the rating of 4.3 and 4.9. In general, plant health declined considerably from the 5th week after M. phaseolina inoculation. Plant health appeared to be slightly better in plants treated with entomopathogenic fungi, but there was no statistically significant difference in any except one instance. Plants treated with M. anisopliae one week prior to the application of M. phaseolina had a rating of 3.0 compared to 1.6 rating of plants inoculated with M. phaseolina alone.
When data from different treatments for each entomopathogenic fungus were compared, both B. bassiana and M. anisopliae s.l. appeared to reduce the wilting, but the plant health rating was not significantly different from the M. phaseolina treatment alone.
This is the first report of the impact of entomopathogenic fungi on M. phaseolina with some promise. Additional studies under more uniform environmental conditions and with more treatment options would shed more light on this approach of using entomopathogenic fungi against M. phaseolina. The current study evaluated single application of the entomopathogenic fungi and we plan to conduct additional studies with multiple applications.
Acknowledgements: We thank Dr. Kelly Ivors (previously at Cal Poly San Luis Obispo) for the pathogen inoculum and Dr. Stefan Jaronski, USDA-ARS, Sidney, MT for multiplying the entomopathogenic fungal inocula.
References
Dara, S. K. and D. Peck. 2017. Evaluating beneficial microbe-based products for their impact on strawberry plant growth, health, and fruit yield. UC ANR eJournal Strawberries and Vegetables. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=25122
Dara, S. K. and D. Peck. 2018. Evaluation of additive, soil amendment, and biostimulant products in Santa Maria strawberry. CAPCA Adviser, 21(5): 44-50.
Dara, S. K., S.S.R. Dara, and S. S. Dara. 2017. Impact of entomopathogenic fungi on the growth, development, and health of cabbage growing under water stress. Amer. J. Plant Sci. 8: 1224-1233. http://file.scirp.org/pdf/AJPS_2017051714172937.pdf
Dara, S. K., S. S. Dara, S.S.R. Dara, and T. Anderson. 2016. First report of three entomopathogenic fungi offering protection against the plant pathogen, Fusarium oxysporum f.sp. vasinfectum. UC ANR eJournal Strawberries and Vegetables. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=22199
Koike, S. T., G. T. Browne, and T. R. Gordon. 2013. UC IPM pest management guidelines: Strawberry diseases. UC ANR Publication 3468. http://ipm.ucanr.edu/PMG/r734101511.html
Partridge, D. 2003. Macrophomina phaseolina. PP728 Pathogen Profiles, Department of Plant Pathology, North Carolina State University. https://projects.ncsu.edu/cals/course/pp728/Macrophomina/macrophominia_phaseolinia.HTM
Vasebi, Y., N. Safaie, and A. Alizadeh. 2013. Biological control of soybean charcoal root rot disease using bacterial and fungal antagonists in vitro and greenhouse condition. J. Crop Prot. 2(2): 139-150.
- Author: Surendra K. Dara
- Author: Dave Peck, Manzanita Berry Farms
In a continuous effort to explore the potential of additive, soil amendment, biostimulant, and other products, a new study was conducted in a conventional strawberry field at the Manzanita Berry Farms in Santa Maria. The following treatments were administered at different times, from planting till the end of production season, as requested by the manufacturer.
- Untreated control: Other than the soil incorporated fertilizers during the field preparation, no other nutrient inputs were added during the study.
- Grower standard: Transplants were dipped in Switch 62.5WG (cyprodinil+fludioxonil, at 5 oz/100 gal) before planting and a proprietary nutrient regimen that included administration of a humic acid-based product was followed.
- Innovak Global regimen: Nutrisorb-L (a blend of polyhydroxy carboxylic acids) at 28 fl oz/ac, starting 2 wk after planting and every 3 wk thereafter through drip. Packhard (carboxylic acids with calcium and boron) at 28 fl oz/ac, starting at the first fruit set (early January) and every 2 wk thereafter as a foliar spray.
- TerraVesco regimen: A microbe-rich Vermi-extract (worm extract) at 10% vol/vol as a transplant dip for 3 hours, followed by application through drip at 7.5 gal/ac after planting, and again in December, 2017 and January, 2018.
- Fertum regimen: Transplant dip in 1% vol/vol of Germinal Plus (a product from marine algae), followed by drip applications of Booster (a biostimulant and a natural organic fertilizer made from seaweed) at 0.5 gal/ac in late November and late December, 201; Silicium PK (a biostimulant and a natural organic fertilizer based on silicon enriched with phosphorus, potassium and seaweed extracts) at 0.5 gal/ac late December, 2017 and once a month starting from mid February to early July, 2018; and Foliar (a biostimulant and a natural organic fertilizer from marine algae) at 0.5 gal/ac in mid and late January.
- Shemin Garden regimen: EcoSil (a silica fertilizer) at 800 ml/ac once a month starting from early December, 2017 to May, 2018 through drip, and at 200 ml/ac in early May and June, 2018 as a foliar spray; ComCat (based on a plant extract) at 20 gr/ac and EcoFlora (a consortium of Azotobacter spp., Bacillus spp., Paenibacillus spp., Pseudomonas sp., Trichoderma spp., and Streptomyces spp.) at 12 oz/ac one week after EcoSil through drip until May, 2018 and ComCat at 10 gr/ac and EcoFlora at 12 oz/ac as a foliar spray in May and June, 2018.
- GrowCentia regimen-low: Yeti containing 1% bacterial culture (of Pseudomonas putida, Citrobacter freundii, Comamonas testosterone, and Enterobacter cloacae) and 2% alfalfa extract applied at 0.6 ml/gal through drip for 90 min weekly from the first drip application.
- GrowCentia regimen-high: Yeti at 1 ml/gal through drip for 90 min weekly from the first drip application.
- NanoChem regimen: EX10, a biodegradable fertilizer additive containing thermal polyaspartate at 1 qrt/ac through first drip after planting with follow up applications in early January (first bloom), mid February, and mid May, 2018. The active ingredient binds with cations such as ammonium, calcium, copper, iron, magnesium, manganese, potassium, and zinc and improves their availability for the plant.
- BiOWiSH regimen 1: Formula 1 at 1.33 oz/gal for transplant dip followed by 3.53 oz/ac through drip starting 2 wk after planting and every 4-5 wk thereafter.
- BiOWiSH regimen 2: Formula 1 at 1.33 oz/gal for transplant dip followed by 3.53 oz/ac as a foliar srpay starting 2 wk after planting and every 4-5 wk thereafter.
- BiOWiSH regimen 3: Formula 1 at 1.33 oz/gal for transplant dip followed by 3.53 oz/ac through drip starting 2 wk after planting alternated with a foliar spray every 4-5 wk.
- BiOWiSH regimen 4: Formula 1 at 1.33 oz/gal for transplant dip followed by BiOWiSH Crop 16-40-0, a microbial consortium (Bacillus amyloliquefaciens, B. lichenoformis, B. pumilus, and B. subtilis)at 3.53 oz/ac through drip starting 2 wk after planting and every 4-5 wk thereafter.
Each treatment contained a 165' long 5.7' wide bed and replicated four times in a randomized complete block design. A 15' long plot in the center of the bed was marked and netted for collecting yield and some other parameters that were compared. Strawberry cultivar BG 6-30214 was planted on 7 November, 2017. Other than the untreated control, all other products were administered on top of the grower standard fertility program. However, only the grower standard transplants were dipped in Switch 62.5WG before planting.
Various parameters were measured during the vegetative growth and fruit production periods to evaluate the impact of the treatments on crop growth, health, and yield. Data were analyzed using ANOVA and LSD test was used to separate significant means.

Transplant treatment (above) and drip application (below). Photos by Tamas Zold
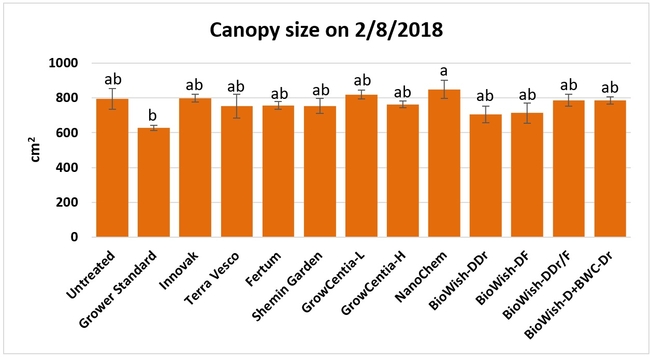
Canopy growth: Canopy growth was observed on 11 December, 2017, 7 and 30 January, and 8 February, 2018 by measuring the size of the canopy along and across the length of the bed from 20 random plants per bed and calculating the area. Canopy size significantly (P = 0.0261) different among the treatments only on the last observation date where plants treated with EX10 and the GrowCentia product at the low concentration were larger than those in the grower standard.
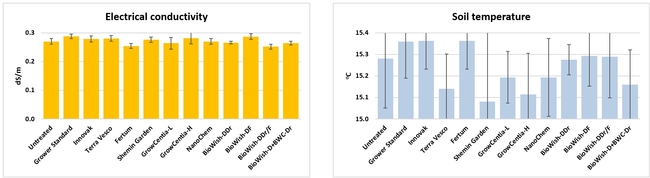
Electrical conductivity and temperature of soil: From two random location on each bed, electrical conductivity (EC in dS/m) and temperature (oC) were measured about 3 inches deep from the surface on 12 and 25 January, 7 February, 19 March, 18 April, and 29 May, 2018. Only soil temperature on 25 January significantly (P = 0.0007) varied among treatments where the difference between the highest (untreated control) and the lowest (Vermi-extract) values was 0.8oC.
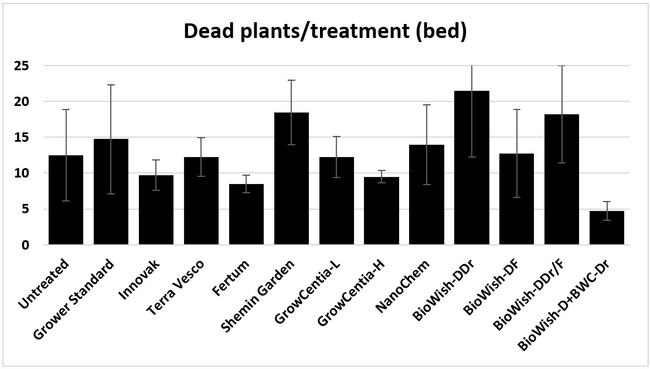
Dead plants: The number of dead plants represents empty spots in the bed due to the death of transplants. There were no obvious signs of disease or a particular stress factor associated with those plants except that they were randomly distributed within each bed and throughout the field. When counted on 18 April, 2018, BiOWiSH regimen 4, Fertum regimen, GrowCentia product at the high rate, and Innovak Global regimen had
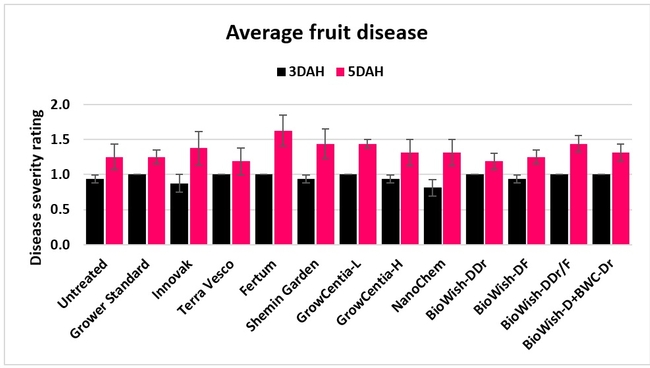
Fruit diseases: Fruit harvested on 12 March, 3 and 13 April, and 17 May, 2018 from each marked plot was incubated at room temperature in dark in plastic containers and the fungal growth was rated 3 and 5 days after harvest (DAH) using a scale of 0 to 4 where 0=no fungal growth, 1=1-25%, 2=26-50%, 3=51-75%, and 4=76-100% fungal growth. Botrytis fruit rot or grey mold was predominant during the first two observation dates and the growth of other fungi (possibly Rhizopus spp.) was also seen during the last two dates. In general, fruit disease occurred at low levels throughout the observation period with
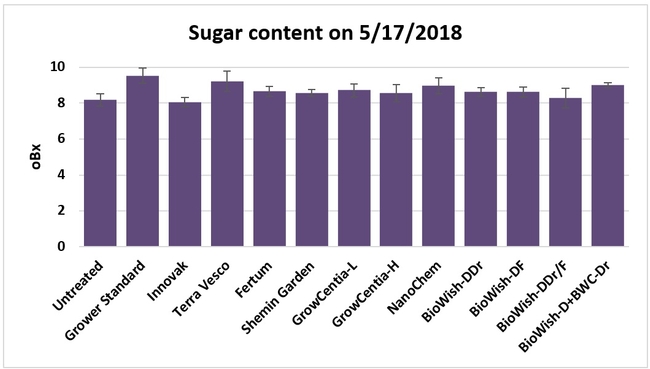
Sugar content in fruit: Sugar content was measured from two harvest-ready berries per bed on 17 May, 2018 using a handheld refractometer. Sugar content varied from 8.06 oBx (Innovak Global regimen) to 9.53 oBx (grower standard).
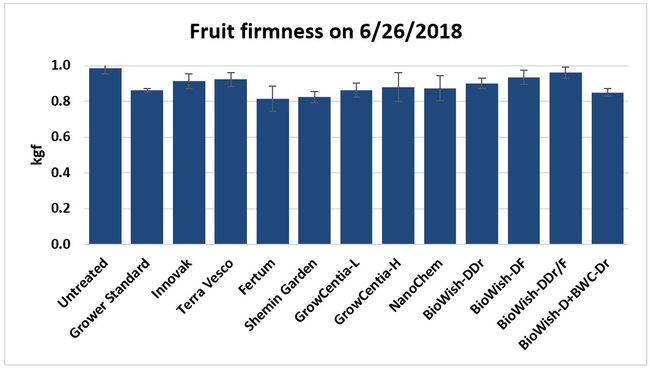
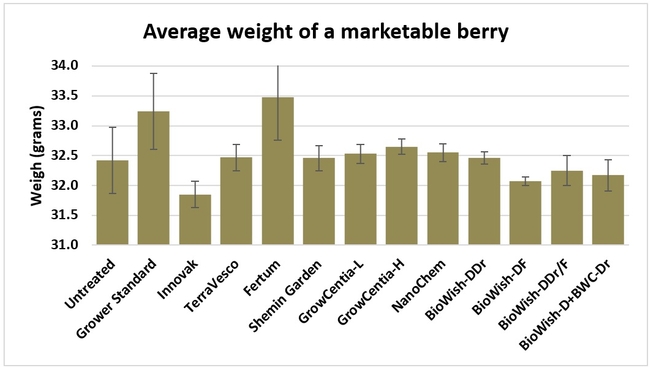
Fruit firmness: Fruit firmness was measured from eight randomly collected harvest-ready berries from each bed on 28 June, 2018. Firmness varied from 0.82 kgf (Fertum and Shemin Garden regimens) to 0.98 kgf (untreated control).
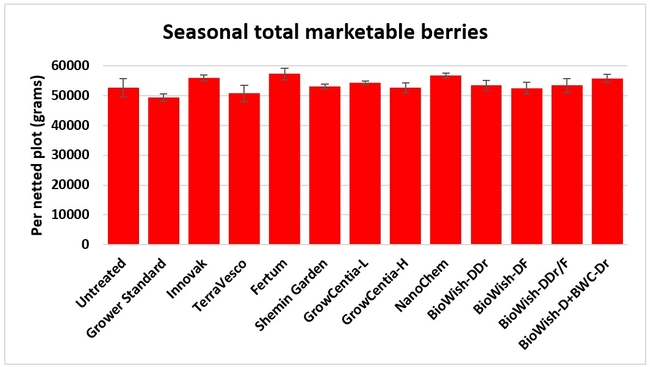
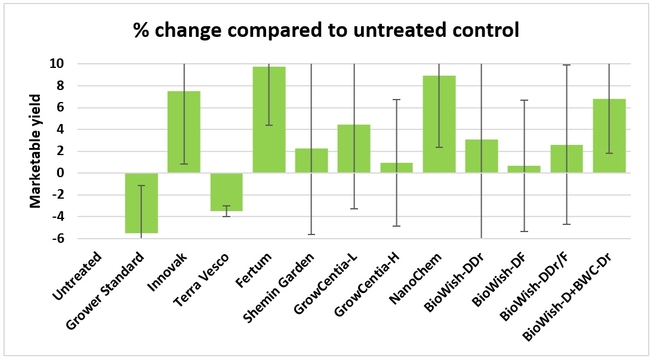
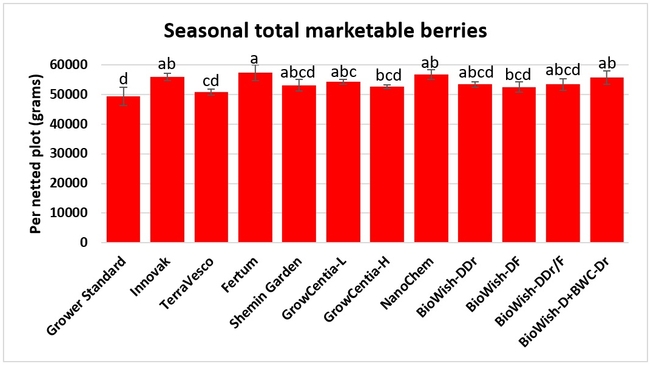
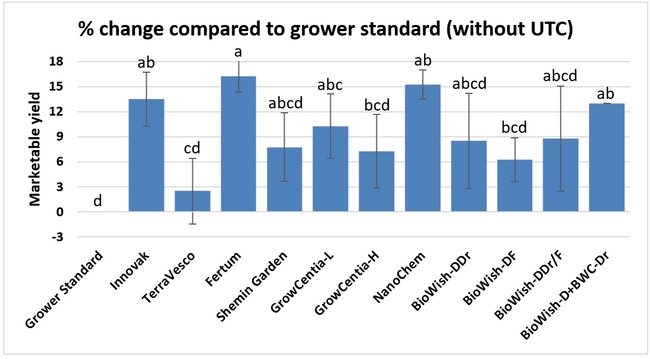
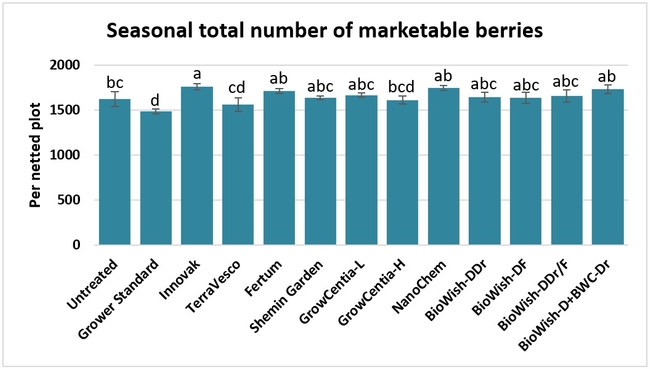
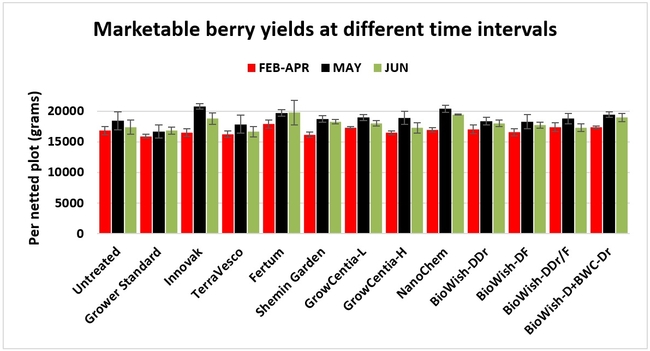
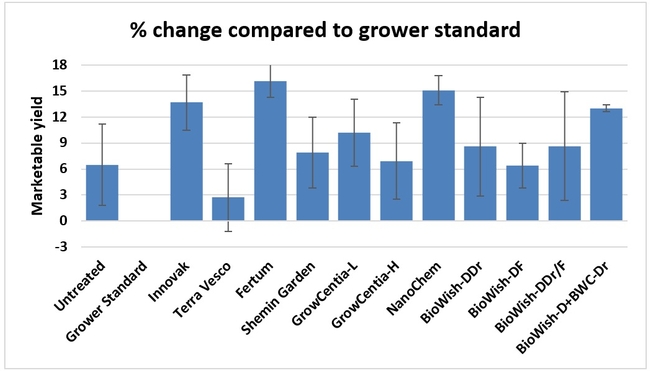
Fruit yield:Strawberries were harvested from 6 February to 22 June, 2018 on 36 dates. When compared to the grower standard, the marketable berry yield was 16.2, 15.1, 13.7, and 13% higher in Fertum regimen, EX10 treatment, Innovak Global regimen, and BiOWiSH regimen 4, respectively. The marketable berry yield was 9.8, 9, 7.5, and 6.8% higher in those respective treatments over the yield from untreated control.
It took 23 harvest dates in three months (from February to April, 2018) to obtain the first third of the total seasonal yield while the remaining two-thirds were obtained from seven harvest dates in May and six dates in June. Marketable fruit yield was higher than the grower standard in all treatments and higher than the untreated control in most treatments.
In general, fruit yields were higher and the pest and disease pressure was lower than usual during the study period. Aleo, a garlic oil based fungicide, at lower label rates was periodically used for disease management and bug vacuums were operated a few times against the western tarnished plant bug as a standard across all treatments.
This study evaluated some treatment regimens as recommended by the collaborating manufacturers and some of them appear to have a potential for use in strawberry production. These results help the manufacturers fine tune their recommendations for achieving better yields through additional studies.
Acknowledgments: We thank the planting and harvest crew at Manzanita Berry Farms for their help with the crop production aspects, Chris Martinez, Tamas Zold, and Maria Murrietta for their technical assistance, Sumanth Dara for statistical analysis, and the support of the industry collaborators who funded the study.