All Issues
Incorporating straw may induce sulfide toxicity in paddy rice
Publication Information
California Agriculture 57(2):55-59. https://doi.org/10.3733/ca.v057n02p55
Published April 01, 2003
PDF | Citation | Permissions
Abstract
Sulfide toxicity to rice plants has been randomly observed in isolated sites in rice fields and experimental plots in the Sacramento Valley. Plants suffering from sulfide toxicity show signs of retarded growth and reduced yields, including the characteristic blackened roots and, in the most severe cases, death. Because the environmental conditions causing sulfide toxicity are not clear, we carried out a greenhouse pot test. The treatments included straw and sulfate additions to a rice soil to induce sulfide production. Our results contribute to an improved definition of conditions leading to sulfide production, toxicity and impact to rice plants.
Full text
In 2001, about 470,000 acres of rice (Oryze Sativa L.) were grown in California, mainly in the Sacramento Valley. Traditionally, rice straw has been burned after harvest to ease tillage and to control rice stem rot. But recently, the burning of rice straw has been restricted in California to improve air quality under the Connelly-Areia-Chandler Rice Straw Burning Reduction Act of 1991 and Senate Bill 318 in 1999. The purpose of these acts was to improve air quality.
Because commercial uses of rice straw are limited, rice growers dispose of the straw they cannot burn, most often by incorporating it into the soil (Bird et al. 2002). Rice researchers are concerned that over the long term, as incorporated straw decomposes in the soil, it might produce constituents toxic to rice, such as sulfide. Plants suffering from sulfide toxicity show signs of retarded growth and reduced yields, including the characteristic blackened roots and, in the most severe cases, death.
Above, Sacramento Valley rice fields. Right, plants with healthy roots (left) and blackened roots (right); the latter is caused by sulfide toxicity.
UC Cooperative Extension rice farm advisors have noted random, isolated cases of what appears to be sulfide toxicity in the Sacramento Valley. Field observation shows that the roots of affected plants are blackened from the accumulation of iron sulfides (and possibly manganese sulfides) formed under reducing conditions. This black coloring disappears upon exposure to the atmosphere after several hours due to oxidation, a confirmation of sulfide formation. Because the soil conditions producing sulfide and sulfide toxicity to rice plants when straw is incorporated are not clear, we conducted a greenhouse pot study.
Reactions driven by microbes
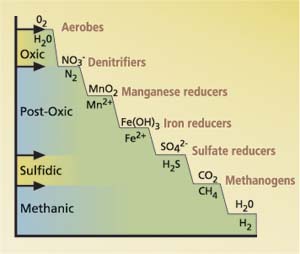
As rice straw and other soil organic matter decompose in submerged soil, a sequence of reduction-oxidation (redox) reactions occur that are driven by a variety of microbes (fig. 1) This sequence of microbially mediated reactions is shown as a redox ladder. The oxygen sufficiency of an environment can be indicated by its redox status, which can be roughly indexed by redox potential (Eh) as measured by a platinum and reference electrodes. A high Eh represents oxic (oxygen-rich) conditions and a low Eh represents anoxic (oxygen-poor) conditions. Redox status can also be classified into oxic, post-oxic, sulfidic and methanic status, as different redox reactions occur that correspond to decreases in Eh.
Microbes decompose organic matter to obtain energy, and they need oxygen or other oxidized substances such as nitrate (NO3-), manganese (Mn [IV or III]), iron (Fe [III]), sulfate (SO4-2) or carbon dioxide (CO2) to serve as electron acceptors. In submerged paddy soil, the decay of organic matter will initially consume the dissolved oxygen in water. Dissolved oxygen (O2) which is an electron-poor substance is reduced to an electron-rich substance (H2O) because it serves as the electron acceptor while organic matter is being oxidized. (This is an example of a redox reaction.) When oxygen is depleted, microbes reduce nitrate to nitrogen gas (N2) (denitrification), and convert the soil from oxic to post-oxic status. In post-oxic status, organic matter continues to decay, and oxidized manganese and iron present in solid phases are reduced to soluble manganous (Mn2+) and soluble ferrous (Fe2+) ions. The continuous decay of organic matter leads to sulfidic conditions, and microbes reduce sulfate to sulfide. Under highly reducing condition (fig. 1) microbes (methanogens) reduce carbon dioxide to produce methane. This process is defined as methanogenesis and the resulting condition is termed “methanic status.”
Fig. 1. Redox reactions catalyzed by microbes during decay of soil organic matter and geochemical redox classes.
Under post-oxic conditions, soluble manganous and soluble ferrous ions are produced; under sulfidic conditions, dissolved hydrogen sulfide (mainly as HS-) is produced. When sulfides accumulate, they may precipitate out as ferrous and manganous sulfides (such as FeS and MnS), reducing the accumulation of soluble sulfides to very low concentrations. But if soluble ferric and manganic sources are depleted, sulfide may accumulate. As a result, sulfide accumulation and toxicity to rice plants may occur only under specific environmental conditions.
Greenhouse study
Experimental soil (Willows clay) was obtained from a burned-straw treatment plot at the UC Rice Straw Management Project near Maxwell. This soil is moderately alkaline. The extract from soil saturation paste had a neutral pH, electrical conductivity (EC) of 2.5 deciSiemens/meter (dS/m) and sulfate concentration of 16 milliMole/liter (mmol/L). Using the Loeppert and Inskeep method (1996), we measured the content of amorphous iron and manganese oxyhydroxides in this soil at 110 milliMole/kilogram soil (mmol/kg) and 8.0 mmol/kg, respectively.
Three levels of straw chopped into 1-inch lengths were mixed into the soil at 0, 6 and 23 tons/acre (0%, 0.6% and 2.3%, respectively). The typical annual straw incorporation in unburned rice fields is about 3 to 4 tons/acre, and any partly decomposed straw may be carried over each year (Bird et al. 2002). The purpose of choosing a much higher straw incorporation rate of 23 ton/acre was to examine the potential damage of straw return on rice with only one growing season of treatment.
The straw-incorporated soil (9.5 pounds) was placed in PVC pots (8 inches in diameter and 8 inches in height). Ammonium sulfate [(NH4)2SO4] was added at rates of 0, 350 and 1,820 pound/acre (0, 160 and 800 milligram/kilogram (mg/kg), respectively) to ensure that sufficient sulfate was available for reduction to sulfide. The fertilization rates were 530 pound/acre of nitrogen as ammonium sulfate and urea, and 64 pound/acre of potassium and 37 pound/acre of phosphorus as potassium phosphate, monobasic (KH2PO4) and potassium phosphate, dibasic (K2HPO4). Our nitrogen fertilization rate was about three to five times greater than normal field applications (Bird et al. 2002) to ensure that the rice plants did not suffer from nitrogen deficiency due to high rates of added straw, and that nitrogen use was not a variable in the treatments.
Microbes decomposing straw require nitrogen as a nutrient competing with nitrogen uptake by plants. Urea and ammonium are oxidized into nitrate, which can be readily assimilated by microbes during straw decomposition and/or is denitrified to form nitrogen gas, causing nitrogen loss from soil (fig. 1) (Bilal et al. 1979). The nitrate becomes less available for plant uptake, as there is competition between immobilization, denitrification and plant uptake. The treatments included three levels of straw and three levels of sulfate done in triplicate (table 1). The pots were flooded on June 27, 2000, and 10 seeds of rice (M202 variety) in germination were transplanted into each pot the following day.
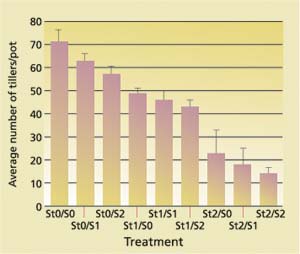
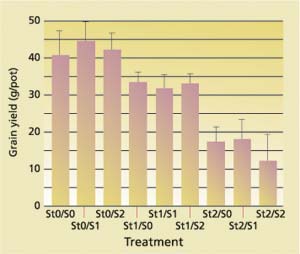
At early growing stages, the 23 ton/acre straw treatment severely affected the rice plants ( see photo, p. 58 ). At week 6 of the growing season, the average number of tillers (extra stems or culms in a rice plant that arise from its base) per pot decreased with increasing straw incorporation as well as with increasing sulfate additions (fig. 2). Likewise, grain yield decreased with increasing straw incorporation (fig. 3).
An analysis of covariance using the GLM procedure and Type II sum of squares was performed using the SAS program (SAS Institute 1991). Statistics on the number of tillers showed that the differences among the three levels of straw treatment were all significant, as were the differences between no sulfate and the highest sulfate addition (P < 0.0036). The grain yield variation from straw treatment effects was significant (P < 0.0001), and the variations in grain yield due to sulfate addition and interaction between straw and sulfate addition were not significant. The highest straw treatment exhibited blackened roots as well as a rotten egg odor characteristic of sulfide.
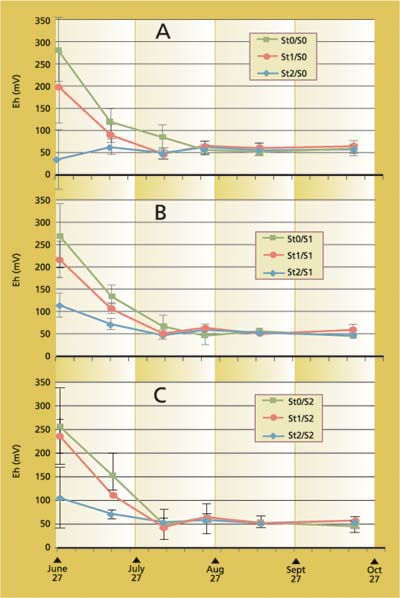
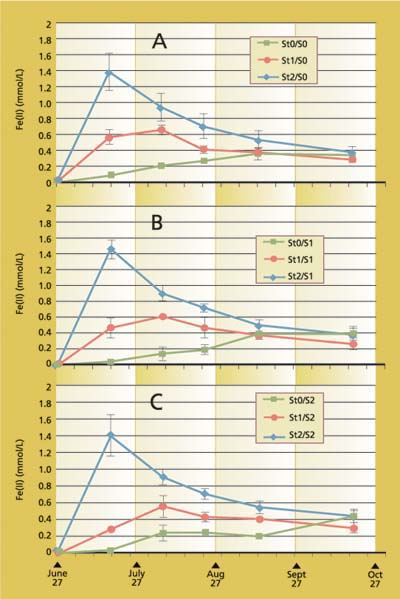
Fig. 4. Redox potential (Eh) at (A) 0, (B) 350 and (C) 1,820 pound/acre sulfate (SO4), and 0, 6 and 23 ton/acre rice straw incorporation.
Fig. 5. Ferrous iron concentration at (A) 0, (B) 350 and (C) 1,820 pound/acre sulfate (SO4), and 0, 6 and 23 ton/acre rice straw incorporation.
Chemical monitoring
The soil solution in the pots was monitored throughout the growing season for redox status and chemical constituents. Soil solution was extracted by vacuum with online monitoring of Eh and dissolved oxygen (DO). For analysis of soluble manganous and ferrous ions (Fe2+ and Mn2+), samples were immediately acidified. For sulfide analysis, samples were immediately treated with a reducing agent to prevent sulfide oxidation; sulfide concentration was determined using an ion-selective electrode (Model 9416; Orion Research Inc.).
The redox potentials (Eh) decreased rapidly upon flooding, and they stabilized by mid-growing season (fig. 4). Note that initially measured Eh of the highest straw treatment after 24 to 48 hours of flooding was about 100 milliVolts (mV) or lower and for no straw treatment, 250 to 300 mV. Lower Eh values indicate more reducing conditions. An Eh value of 50 to 100 mV is considered reduced (Patrick 1981). Differences among Eh values between the treatments after about 3 weeks of flooding were not readily discernible. The pH of the soil solution was near neutral after flooding (data not shown) and so did not affect Eh values.
Changes in composition of the soil water with respect to soluble iron, soluble manganese, sulfate, sulfide and methane were monitored. (Results are shown for soluble iron and sulfate only.) Ferrous iron concentrations generally increased with time (fig. 5). However, in the 23 ton/acre straw treatment with high sulfate addition, iron peaked at 3 weeks, indicating a more rapid decrease in Eh than other treatments. Eh decreased to a level similar to the other treatments by harvest time.
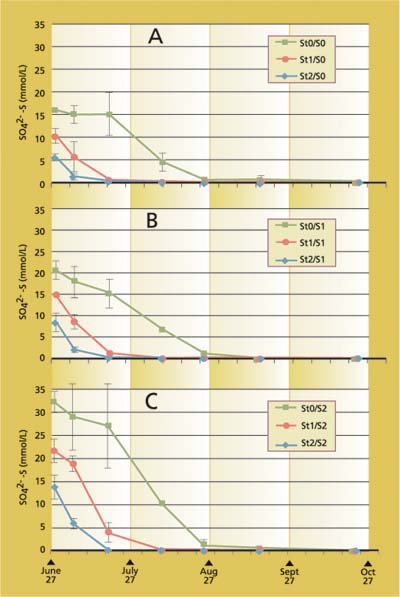
Meanwhile, sulfate concentrations decreased with time to very low concentrations (fig. 6). Initially measured sulfate concentrations were negatively correlated to the level of straw treatment. It is likely that sulfate reduction occurred within the first couple of days in the straw treatment (6 and 23 ton/acre) that resulted in lower sulfate concentrations in the first sampling that was taken after 24 to 48 hours of flooding. Very low concentrations of sulfides were found, however, much lower than expected with the amount of sulfate reduced to sulfide. Most of the samples contained sulfide concentrations below 0.005 mmol/L (data not shown). The low sulfide concentrations were mainly due to formation of ferrous sulfide (FeS) which will be discussed in detail below.
In greenhouse experiments, rice plants responded to straw treatment and sulfate additions. Left to right photos, Treatments with 0, 350 and 1,820 pound/acre sulfate (SO4). Within each photo, pots from left to right contain 0, 6 and 23 ton/acre incorporated straw.
Geochemical redox classes
We defined the redox status as falling within one of several geochemical redox classifications (fig. 1) of the monitored soil solutions. These were based on oxidative capacity, which, in turn, was based on equivalent concentrations of the redox constituents (dissolved oxygen, nitrate, manganic oxide, ferric hydroxide, sulfate and methane) (Gao et al. 2002; Tanji et al. 2001). The no-straw treatment was initially oxic, then post-oxic, and methanic in the last sampling. The 6 ton/acre straw treatments were initially oxic, then sulfidic at 3 weeks, and thereafter methanic. The 23 ton/acre straw treatment rapidly became methanic after an initial oxic status. Straw additions clearly resulted in the fast development of more reduced conditions. We expect sulfide toxicity to rice plants to occur when redox status is sulfidic.
Sulfide solubility, toxicity
This study indicates that elevated levels of straw incorporation may have caused sulfide toxicity effects on rice plants. The most readily apparent visual symptoms of sulfide toxicity are blackened roots, and reduced height and number of tillers. The monitored chemical data did not clearly establish a chemical diagnosis of potential sulfide toxicity. Low sulfide concentrations in soil solutions of sulfide-affected rice plants have been a diagnostic dilemma (Yoshida 1981). We hypothesize that the concentration of sulfides in the soil solution is not a good indicator of sulfide toxicity because of precipitation of sulfides with metals such as iron and manganese.
We tested this hypothesis with a geochemical computer model called WATEQ (Ball and Nordstrom 1991). This model examines the speciation of iron and sulfur in solution and predicts whether or not ferrous sulfide is precipitated based on calculations of saturation indexes. The model predicted that when the FeS was formed, the sulfide concentration was extremely low (< 0.001 mmol/L) and thus difficult to measure. This is why chemical diagnosis of sulfide toxicity is so difficult and rarely produces clear evidence. In the Sacramento Valley, sulfide toxicity is sometimes associated with water salinity, possibly because waters with higher electrical conductivity may contain higher concentrations of sulfate. Undissociated hydrogen sulfide is the toxic form to rice plants (Yoshida 1981). Hydrogen sulfide is a strong inhibitor of aerobic respiration after entering the roots, causing nutritional imbalance and physiological disorders (Kumazawa 1984). This point needs further research. At any rate, the best diagnosis for sulfide toxicity is blackened roots and rotten egg odor (hydrogen sulfide). Reduced height and number of tillers may also be an early sign of sulfide toxicity. In the most severe cases, rice plants die. Incorporating excessively high rates of straw may enhance sulfide toxicity to rice plants, but under current straw return rates, it is unlikely that sulfide toxicity could occur on a large scale. It is more likely that only randomly localized sites will exhibit toxicity.
In this study, the 23 ton/acre straw incorporation significantly reduced rice yield and induced sulfide toxicity symptoms. The 6 ton/acre straw incorporation rate, which is slightly higher than the normal field return rate (4 ton/acre), also reduced rice yield significantly. However, the reduced yield may not be solely attributable to sulfide toxicity. Decomposition of organic matter produces low-molecular-weight organic acids, some of which are toxic to rice plants. Further, it appears that salinity is another factor in reducing rice yield (Scardaci et al. 2002). For example, sulfate additions reduced the number of tillers at the early stage. All these factors may have contributed to rice yield reductions.
Under normal field conditions, straw decomposition after the fall harvest and incorporation can be promoted by winter flooding (Bird et al. 2002). Straw decomposition rates can be slowed down if straw is not well incorporated into the soil and winter flooding is not practiced. Then the organic matter would be subject to decomposition in spring during the rice-growing season. These conditions are more likely to result in sulfide problems. Sulfide problems were also observed in lower basins and on the sides of fields, suggesting that lack of water circulation and/or oxygen may be a contributing factor. Increasing water flow or circulation may therefore ease impacts in some fields.