Walnut Blight Disease Cycle
Walnut blight is caused by the bacterial pathogen Xanthomonas arboricola pv. juglandis (Xaj). This devastating bacterium can overwinter in between scales of healthy buds, “waiting” to be rain-splashed onto the developing flowers and leaves. Twig cankers are another overwintering mechanism that can supply inoculum for primary infection (see photos 1, 2, & 3). Bud infections can result in bud death, and fruit infections sometimes lead to peduncle infections that do not dehisce from twigs. These infections can develop into twig cankers during the growing season, and they represent another mechanism of survival by the pathogen from one season to the next. In a recent study, healthy buds next to twig cankers harbored significantly more bacterial cells than buds not adjacent to twig cankers. This indicates that twig cankers are a source of inoculum for contaminating healthy buds.
Under wet conditions, these cankers can ooze out bacterial cells that can be disseminated to surrounding tissue including healthy buds, flowers, and fruit. These disseminated bacterial cells can then live epiphytically (remain on the surface) or infect green tissues.
At the beginning of the growing season as buds and shoots grow, the epiphytic pathogen can be carried out from between the bud scales onto the new growth. Catkin and pistillate flower infections can arise from primary inoculum in healthy buds or cankers. Fruit infections that arise from the stylar end are classified as “end blight” due to the most common symptom being sunken black lesions at the flower end of the nut (photo 4). This type of infection is characteristic of a primary infection resulting from infection at the stylar end of the female flower. If wet, rainy, conducive conditions for disease exist, twig cankers can also be primary inoculum sources for catkin and pistillate flower, as well as fruit infections. Newly blighted tissues (male and female flowers, fruit with “end blight”) can serve as secondary sources of inoculum. “Side blight” is a typical symptom of secondary infections (photo 5). End blight infections will typically kill the developing kernel resulting in a dropped nut, whereas secondary side blight infections, if they occur later in the season or after the nut is fully formed, do not typically result in a dropped walnut but may predispose the nut to worm damage or result in off-graded kernels.
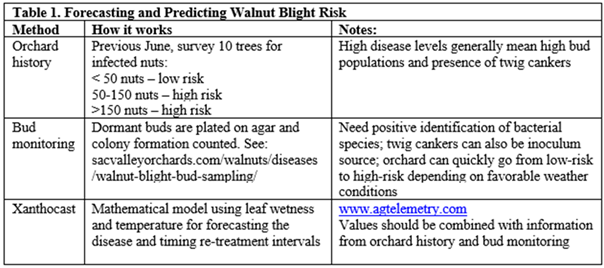
For attempting to predict or forecast walnut blight, utilizing orchard history, bud monitoring and Xanthocast are the three methods (Table 1). All methods have advantages and disadvantages and should be used as information to guide the grower/PCA decision-making process for implementing a preventative management program
Disease management
Kasugamycin (tradename Kasumin) was registered in 2018 for managing walnut blight and bacterial diseases of some other crops. Kasugamycin is a unique bactericide because it is not used in animal or human medicine. Environmental monitoring studies have shown that it does not select for human bacterial pathogen resistance with uses in plant agriculture. Furthermore, kasugamycin has its own FRAC Code 24 or mode of action that is different from other registered plant agricultural bactericides. Kasugamycin meets new toxicology standards for pollinating insects (e.g., honey bees), and it has a low animal toxicity with a “Caution” rating and a 12-hour re-entry time on the label. Still, as with any cautionary pesticide, mixers and applicators need to have standard personal protective equipment (PPE) when handling the bactericide. Thus, the three most effective conventional bactericides now available are copper, mancozeb, and the newly registered kasugamycin. Ratings for these and biological materials can be found in the annually updated Efficacy and Timing of Fungicides, Bactericides, and Biologicals.
Copper is classified as FRAC Code M1 for the first element historically used for fungal and bacterial disease control. Copper affects many physiological pathways in plant pathogens and is classified as having a multi-site (M) mode of action. Not many bactericides have been developed for managing plant bacterial diseases, and fewer have been registered. Thus, there has been a great dependency on copper. Unfortunately, after many years of usage, bacterial pathogens such as the walnut blight pathogen Xaj, have developed resistance to copper. This is a direct result of overuse of one active ingredient (i.e., copper) and being limited with the lack of bactericides available to apply modern approaches to resistance management such as rotating between active ingredients with different modes of action and limiting the total number of applications of any one mode of action per season as part of following resistance management best practices (see “RULES” on pg. 9). Over-usage of any one active ingredient can create other environmental issues including possible soil and water contamination, as well as potential crop phytotoxicity especially in perennial crop systems.
To overcome copper resistance, copper-maneb (e.g., Manex) mixtures were first identified for use on walnut in 1992 and emergency registrations ensued until the full registration was obtained for the related compound mancozeb in 2014. Because copper resistance had already developed, this selection pressure for copper is maintained, and resistance levels are increasing from 50 ppm to over 100 ppm MCE even when mancozeb is used in the mixture. In effect, resistance management is not being effectively practiced since copper-resistance already exists and the use of mancozeb (M3) is selecting for resistant strains of the bacterial pathogen to the mancozeb mode of action. Without different modes of action to put into a rotation, resistance to mancozeb is inevitable unless new modes of action are registered that can be used in rotation with copper-mancozeb treatments.
Kasugamycin was identified, developed, and registered for the purpose of resistance management, reducing over-usage of any one mode of action, and sustaining the walnut industry of California. The bactericide has a unique mode of action and should be used in combination with copper or mancozeb. When kasugamycin is used in combination with mancozeb, good resistance management is being practiced since resistance has not been found in Xaj populations to either mancozeb or kasugamycin.
Kasugamycin Use on Walnuts
Kasugamycin is labeled as Kasumin for managing walnut blight and some of the label restrictions and guidelines are shown in Table 2. Applications should be initiated when conditions favor disease development. This is the same timing as for copper-mancozeb. In orchards with a history of the disease and when high rainfall is forecasted, applications should be initiated at 20-40% catkin expansion. Under moderate/low disease pressure (i.e., low rainfall forecasts and minimal dews), applications should start at 20% prayer stage (leaflets unfolding, before expansion), and at 40 % prayer stage when disease pressure is very low. These stages correspond to pistillate flower emergence.
The best way to use the bactericide is in combination with mancozeb or copper. Kasugamycin-mancozeb mixtures applied in our research trials were often the most effective of all treatments evaluated. Sample application management strategies for a two-, four- or five-spray rotational program are shown in Table 3.
Suggested programs include a re-application interval of 7 to 10 days. The reason for this re-application interval is that most non-metal bactericides have a short residual life of a few days to a week or two and that Kasumin is locally systemic or translaminar and thus, is less likely to be re-distributed.
Having copper-mancozeb last in the rotation will also provide the longest lasting residuals of both active ingredients. Furthermore, with new growth increasing the canopy volume weekly in the spring as walnut trees come out of dormancy, multiple and frequent applications are necessary for most cultivars flowering and fruiting in potentially high rainfall periods.
Kasugamycin and resistance
Resistance is a relative term indicating a change in sensitivity to an inhibitory compound. A moderately high minimal inhibitory concentration (MIC) for a bactericide does not mean that the pathogen is resistant. We have conducted baseline studies with kasugamycin, kasugamycin-copper, and kasugamycin-mancozeb for Xaj with MIC values of 20, 8.3, 5.3 ppm, respectively (the lower the value, the more toxic the chemical or chemical mixture). This was done before the bactericide was registered in California to determine any change in sensitivity after registration and commercial usage can be assessed. To date, resistance has not been found and isolates evaluated are all within the baseline distributions. Still, if resistance management strategies are not employed, there is a risk for selecting for pathogen resistance to kasugamycin. This is the reason why we developed the mixture-rotation programs suggested above.
Host Resistance
Cooperative research on walnut blight between UC Riverside and the UC Davis breeding program has led to several ways to evaluate new genotypes for blight resistance. The standard way is using natural incidence of walnut blight on trees that are old enough to have developed enough fruit that can be evaluated. This takes four to five years. Another method is to inoculate fruit, allowing susceptibility data to be collected regardless of rainfall events. Still, we have to use fruit for this assay and thus, the timeline for obtaining information on host susceptibility is not shortened. The third approach we are using for evaluating new genotypes for blight resistance is to inoculate buds at the beginning of the season and determine if the buds can support a pathogen population until the end of the season. Results show that many of the genotypes that support a high bud population also have a high incidence of fruit infections. Still, there are a few genotypes that do not support a bud population, and yet have high disease. These genotypes may have other ways for the pathogen to survive, such as cankers. Genotypes with traits that both restrict survival of the pathogen in their buds and have low fruit disease can be further advanced in a breeding program.
Conclusions
The integration of bactericides with different modes of action, application strategies of rotations of mixtures of bactericides with different modes of action, and forecasting tools such as XanthoCast (agtelemetry.com) should provide the stewardship necessary for having the tools available for managing walnut blight for years to come. The anticipation with the Kasumin registration is to provide resistance management and prevent or reduce the risk of resistance to copper-mancozeb while new approaches can be developed and integrated to protect kasugamycin and mancozeb from further resistance selection in pathogen populations. The industry needs new bactericides and several new modes of action are under development and in the process of registration. Walnut blight is the most serious disease impacting growers in California and multiple tools like kasugamycin, copper, and mancozeb need to be available to maintain a successful industry.
Common Mistakes with Walnut Blight Control:
- First spray timing too late.
- Blight population increased resulting in high disease pressure.
- Material rates too low.
- Poor spray coverage both by air and ground.
- Using a weak material in high blight potential orchards.
- Half sprays from every-other-row application.
- Not tank mixing.
- Dense tree canopies.
Author - Tree Crop and Environmental Horticulture Advisor Emeritus, Master Gardener Advisor