- Author: Brad Hanson
Glyphosate is one of the most widely used herbicides in the world and is extremely important in many of our orchard, vineyard, and annual crops as well as in non-crop and home situations. However, it can be confusing to understand some of the differences among various formulations of glyphosate herbicides.
I'll paraphrase a recurring extension question as “I'm trying to compare the rates and cost-effectiveness of two glyphosate herbicides. One lists the active ingredient as ‘41% glyphosate as the isopropylamine salt' and the other as 48.7% glyphosate as the potassium salt'. How do I compare these two herbicides?”
First important point, glyphosate is a weak acid herbicide. The various salt formulations have major impacts on how the herbicide behaves in the jug, and to some degree on how it gets into the plant. But once in the plant, it is the glyphosate acid that binds to the target enzyme in susceptible plants and causes the herbicidal effect.
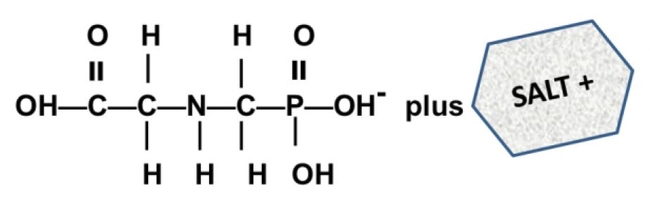
What is a salt? From a chemistry perspective, a salt is simply a compound formed by ionic bonding of two ions of opposite charge. Glyphosate acid has a weak negative charge and the salt is formed when the glyphosate acid is bound to a base that has a positive charge. In the cartoon below, this is illustrated a little incorrectly - you can see the negatively charged glyphosate acid on the left (C3H8NO5P); however, the "salt+" tagged on the right should really be labeled "base+" (the combined molecule is actually the "salt").
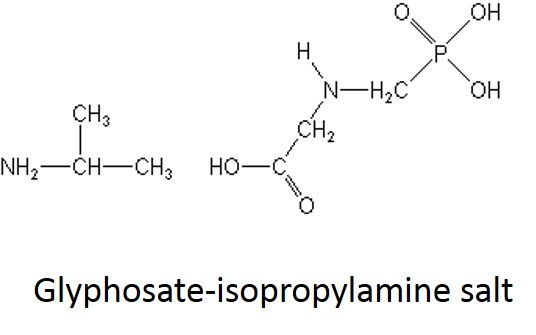
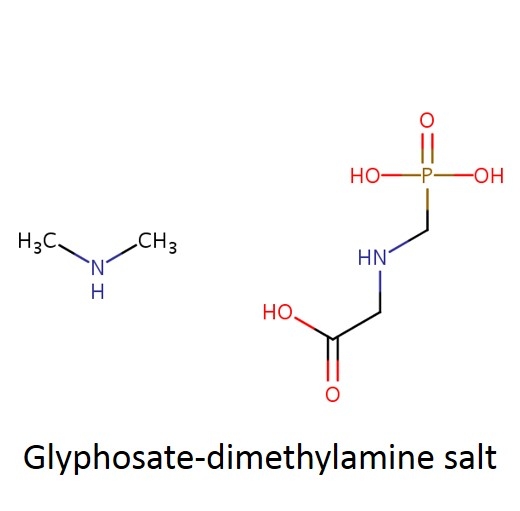
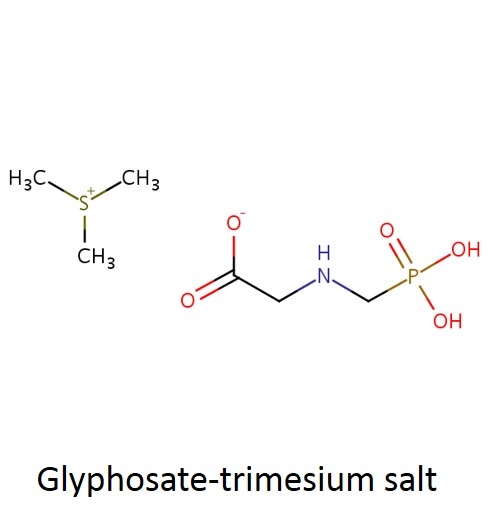
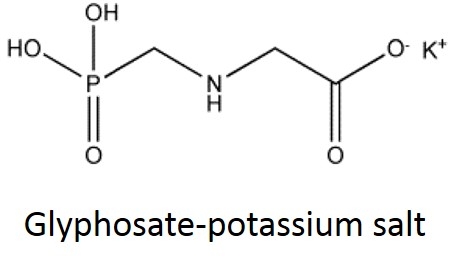
What are some common glyphosate salts? There are several glyphosate salts currently available in the market and others have been in the past but are less common now. Four examples (below) include: isopropylamine, dimethalamine, trimesium, and potassium salts. (images mostly lifted and modified from www.chemsrc.com).
In case it's been a while since your last organic chemistry class, here's a quick refresher on nomenclature. In the isopropylamine salt, that simply means it as a 3-carbon chain (that's “propyl”) and amine group (NH), and the chain is connected in the middle (the “iso” position) rather than on one end. Dimethylamine indicates two (di) methyl groups and an amine. Trimesium is a shortened name for trimethylsulfonium which means three methyl groups and a sulfur. Potassium means, well that one's pretty straightforward and means potassium (K).
How does the salt formulation affect the herbicide? The salt formulations are those of us managing weeds for a couple of important reasons.
- First, the different glyphosate salts have different solubility in water (or other solutions). This doesn't have much, if any, effect when we have the herbicide diluted in water to make a spray application (eg. quarts of product in 10 or more gallons of water). However, it has a big impact on how concentrated the herbicide can be in the formulated product. (It's useful here to note that pure glyphosate is actually a solid crystal that is dissolved in liquid to make the products we use in the field.) In the example I started with, that is the big driver behind why one product has 41% active ingredient and the other has 48.7%. From a packaging, shipping, storing, and handling perspective, it's far more efficient to have more concentrated materials (eg less water). So, we can assume the potassium salt is quite a bit more soluble than the isopropylamine salt form of this herbicide.
- Second, the different glyphosate salts have different weights. Remember the periodic table of the elements? The atoms that make up the salts (and any other chemical structure for that matter) have markedly different weights on an atomic scale. For our purposes, we can think of this as a carbon (C, atomic number 6) weighing six times a hydrogen atom (H, atomic number 1) and a potassium (K, atomic number 19) weighs about three times what carbon does. Of course, the glyphosate acid weight is the same C3H8NO5P in each formulation – only the salt is different. So, it is important to remember that in these herbicides the “active ingredient” (AI) is the salt formulation (eg glyphosate isopropylamine) and that each of the different active ingredients has a slightly different weight.
How can I use the percent active ingredient to compare products? I'll be honest here, I find it very confusing to think about the percent AI list on the label of a glyphosate herbicide. As I indicated above, this does not allow a straight across comparison for two different salts (eg a potassium salt glyphosate is not the same weight as a dimethylamine salt). More importantly, the percentage listed on the label is actually on a weight basis (weight of glyphosate salt per weight of formulated herbicide). I don't know about any of you, but I typically measure glyphosate herbicides by volume (fluid ounces, quarts, milliliters) rather than by weight (ounces, pounds, kilos). So knowing the amount of glyphosate per pound of Roundup Powermax isn't very helpful to me when I don't weigh the liquid.
Instead of focusing on the percent active ingredient, look on the label just below where the percentages are listed for the active ingredient and acid equivalent information. (I'm looking at a Roundup PowerMax label and a Credit41 label, but there are dozens of examples).
In these two herbicides, you can see:
- Credit41 has 4 lbs per gallon of glyphosate as the isopropylamine salt, which is equivalent to 3 lbs per gallon of glyphosate acid.
- Roundup PowerMax has 5.5 lbs per gallon of the glyphosate potassium salt, which equates to 4.5 lb/gal of the acid.
So, once we're comparing on an acid equivalent (AE) basis, we can see that that one product is 50% more concentrated than the other (3 lbs vs 4.5 lb ae). If you wanted to make an equivalent rate (AE per acre) you'd have to apply 50% more of the 3 lb product to have the same amount of glyphosate acid. Similarly, in making cost comparisons, you should be thinking about it in terms of cost per unit of glyphosate acid. In the above example, if making an equivalent AE rate per acres, the breakeven point would be if the 3 lb material costs 2/3 of what the 4.5 lb material costs. Don't make the mistake of assuming a quart of one glyphosate is the same as another (or that a 2% solution of two different products is equivalent!
Check out this publication we did a couple years ago On the second page, there's a chart that illustrates this concept – if you wanted to apply a rate of ¾ lb ae per acre, you'd need to apply 32 fl oz of a 3.0 lb ae/gal material (eg Credit41, Roundup Original) but only 22 fl oz of a 4.5 lb ae/gal material (eg Roundup PowerMax).
What else is different among the formulations? This is a really good question without a really clear answer.
Besides the glyphosate-salt that makes up the active ingredient in each formulation, there are other components of the herbicide product. Some of these influence physical properties that affect handling, storage stability, etc. Others are surfactants and other adjuvants that impact how the herbicide penetrates the leaf surface. The surfactants (types and loading) are proprietary information and are not reported in the same way as the active components are so this can be difficult to ascertain. However, one way that price points can be reduced in this competitive market (lots of generic glyphosate herbicide due to loss of patent protection) is to reduce or change the surfactant packages. Thus, even if the active ingredient is the same between two products, they could have substantially different surfactant loading which can impact weed control efficacy (amount of herbicide getting to the target enzyme in the plants). If you use a low-surfactant-load glyphosate product, you should consider adding appropriate surfactants to make up for it.
I'll wrap this up by saying that I think a weed manager can get similar levels of weed control performance with the wide range of glyphosate herbicides currently available on the market. However, it is really important to make sure you're comparing apples-to-apples in determining rates needed from the various salt formulations and concentrated products. In my experience, at equivalent AE rates, a 3 lb glyphosate plus a good surfactant can perform similarly to a higher AE and higher surfactant load formulations; however, be sure to sharpen your pencils to make sure the savings from the cheaper AI isn't offset by higher required rates and surfactant additions.
12/21/17 - lightly edited to clarify that the "salt" is the combined molecule that includes the glyphosate acid and the "base" (it's been a while since my last O-chem class too!). BH




really it is very interesting but still u didn't answer the differences between the different type of salts in the mod of action . yes the acids is the active parts doing the job .but the different type of salts behaving different in the penetration , solvability , evaporation etc.. , this why the price are different even the performance is different even if u add same additive's [ surfactant and other crier ] . if your assumption is wright , all glyphosate formulators will go for the cheep salts and adding good additives .
Can you please tell which surfactant can we use for better results.
Like poly oxy ethylene amine. Please suggest other surfactants.
[link deleted by moderator]