- Author: Christian Nansen, Elvira de Lange, Alison Stewart, Department of Entomology and Nematology, UC Davis
- Author: Mark Edsall, California Strawberry Commission
- Author: Surendra K. Dara
The western tarnished plant bug (or lygus bug) and different species of spider mites are major arthropod pests in California strawberries. While predatory mite releases are very popular for controlling spider mites in both organic and conventional fields, a significant amount of chemical pesticides are used for arthropod pest management in conventional fields. Nearly 280,000 pounds of active ingredient of at least 30 chemical and botanical pesticides were used in 2016 in California strawberries (USDA-NASS, 2016; CDPR, 2017) and malathion, bifenazate, naled, acequinocyl, and fenpropathrin were the most common of about 79,000 pounds of chemical pesticides. A uniform and thorough coverage of pesticide sprays is essential for effective pest management and also for reducing excessive pesticide use that could lead to resistance and environmental health issues (Shi et al., 2013). Evaluation of pesticide spray applications and their performance will help improve current pest management strategies in strawberry as they did in other crops (Nansen et al., 2011; Nansen et al., 2015).
Several factors such as the tractor speed, spray nozzles, spray volume, boom height, adjuvants, pressure, canopy characters, micro and macroclimatic conditions influence the spray coverage. A better understanding of these factors will help improve the pesticide use and efficacy, optimize the cost, and reduce pesticide drift and other associated risks (Nansen and Ridsdill-Smith, 2013).
A study was conducted during 2016 and 2017 to evaluate multiple spray configurations under varying weather conditions where more than 4000 data points were collected. Data for only two spray configurations, using Albuz ATR 80 Lilac and Albuz ATR 80 Green nozzles, are shown here.
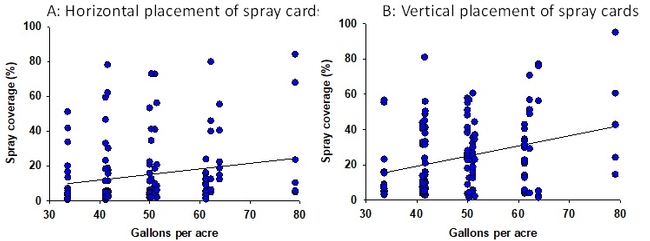
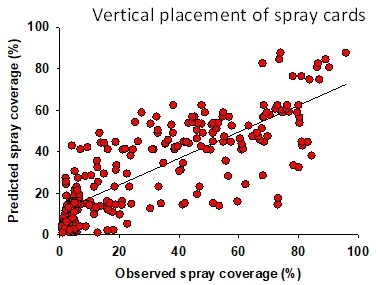
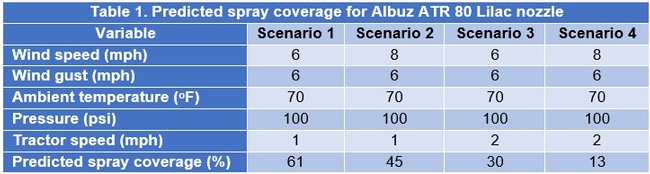
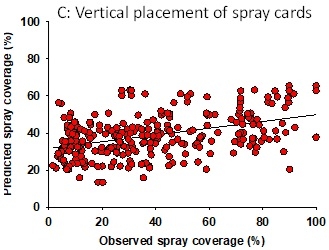
Configuration 1: Albuz ATR 80 Lilac nozzles were used in 144 experimental applications delivering 32-80 gallons of spray volume per acre. Water-sensitive spray cards (TeeJet, Wheaton, IL) were clipped to the petioles of strawberry leaves in horizontal and vertical orientation (1 card per application for each orientation). They were placed in the strawberry canopy prior to spray applications and the coverage was determined based on the pattern on the cards using the SnapCard smartphone application. Data suggested that spray volume does not always translate into a good spray coverage (Fig. 1). There was a wide variation in the coverage that ranged from 0-55% at 33 gpa and 5-80% at 80 gpa suggesting the influence of other factors. Taking the operational and weather conditions into account, a multiple linear regression analysis was conducted to measure the relationship between predicted and observed spray coverage which appeared to have a linear correlation (Adjusted R2=0.27; F+52.6; P < 0.001) (Fig. 2). Wind speed, wind gust, ambient temperature, pressure, and tractor speed were used as explanatory variables in this analysis. Based on this regression model, four possible scenarios were developed with predicted spray coverage values (Table 1). A 2 mph increase in the wind speed from 6 mph to 8 mph could reduce the spray coverage from 61 to 45% when the tractor runs at 1 mph or from 30 to 13% when the tractor runs at 2 mph.
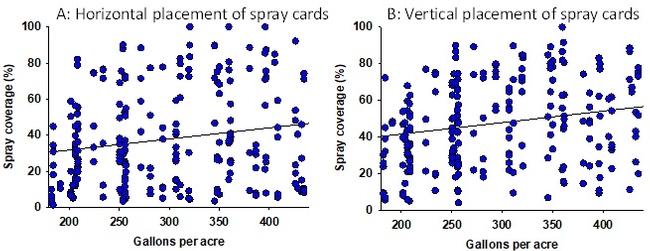
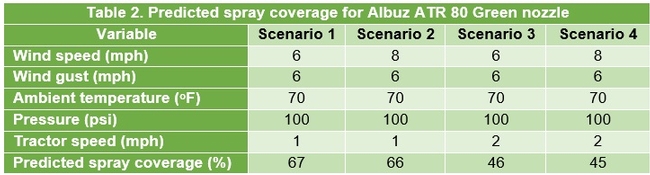
Configuration 2: Albuz ATR 80 Green nozzles were used in 276 experimental applications delivering 180-440 gallons of volume per acre. Since the droplet size from the Green nozzle is much larger than that from the Lilac nozzle, a better coverage is expected with the presumption of lesser sensitivity to environmental and operating conditions. However, data from the spray cards indicated a poor relationship between the spray volume and coverage under this configuration as well (Fig. 3). For example, both 200 and 350 gpa had a similar spray coverage. Multiple linear regression analysis, using strawberry canopy characteristics (plant height and width, dry weight, and canopy coverage), operating and weather conditions as explanatory variables, showed a significant linear correlation (Adjusted R2=0.45; F=199.5; P < 0.001) between observed and predicted spray coverages (Fig. 4). A prediction model under this configuration showed nearly 20% decline in spray coverage when the tractor speed increased from 1 to 2 mph (Table 2). Wind speed appeared to have a minimal impact, probably due to the droplet size.
This study demonstrates the importance of weather and operating conditions on spray coverage. Additional data will be collected in 2018 to expand our understanding of the factors that influence spray coverage. These studies will be useful to determine appropriate operating conditions such as the spray volume, tractor speed, and types of nozzles and identify weather conditions that are ideal to achieve good coverage. A free smartphone application is under development for the growers and PCAs to input weather and operating conditions to predict the spray coverage. This information will ultimately improve the pest control efficacy and contribute to sustainable pest management practices.
Acknowledgments: We thank the financial support of the California Strawberry Commission and the collaboration of several growers. We also thank the technical assistance of Daniel Olivier, Marianna Castiaux, and Ariel Zajdband, California Strawberry Commission and Robert Starnes, Jessie Liu, Laurie Casebier, Haleh Khodaverdi, and Isaac Corral, UC Davis.
References
CDPR. 2017. Summary of pesticide use report data 2016: California Department of Pesticide Regulation, p. 909.
Nansen C, Ferguson JC, Moore J, Groves L, Emery R, Garel N and Hewitt A. 2015. Optimizing pesticide spray coverage using a novel web and smartphone tool, SnapCard. Agronomy for Sustainable Development: 1-11. DOI: 10.1007/s13593-015-0309-y.
Nansen C & Ridsdill-Smith TJ (2013) The performance of insecticides – a critical review: Insecticides (ed. by S Trdan) InTech Europe, Croatia, pp. 195-232.
Nansen C, Vaughn K, Xue Y, Rush C, Workneh F, Goolsby J, Troxclair N, Anciso J, Gregory A, Holman D, Hammond A, Mirkov E, Tantravahi P and Martini X (2011) A decision-support tool to predict spray deposition of insecticides in commercial potato fields and its implications for their performance. Journal of Economic Entomology 104: 1138-1145. DOI: 10.1603/EC10452.
Shi M, Collins PJ, Ridsdill-Smith TJ, Emery RN and Renton M. 2013. Dosage consistency is the key factor in avoiding evolution of resistance to phosphine and population increase in stored-grain pests. Pest Management Science 69: 1049–1060. DOI: 10.1002/ps.3457.
USDA_NASS. 2016. Quick stats.
- Author: Surendra K. Dara
- Author: David Peck, Manzanita Berry Farms
Six-month old strawberry field.
Under the soil is a complex and dynamic world of moisture, pH, salinity, nutrients, microorganisms, and plant roots along with pests, pathogens, weeds and more. A good balance of essential nutrients, moisture, and beneficial microorganisms provides optimal plant growth and yield. These factors also influence natural plant defenses and help withstand stress caused by biotic and abiotic factors.
Several beneficial microbe-based products are commercially available to promote plant growth and improve health, yield potential and quality. Some of them improve nutrient and water absorption while others provide protection against plant pathogens or improve plant defense mechanism. In addition to the macronutrients such as nitrogen, phosphorus, and potassium, several micronutrients are critical for optimal growth and yield potential. Some of the micronutrient products are also useful in promoting beneficial microbes. Understanding the plant-microbe-nutrient interactions and how different products help crop production are helpful for making appropriate decisions.
Mycorrhizae (fungi of roots) establish a symbiotic relationship with plants and serve as an extended network of the root system. They facilitate improved uptake of moisture and nutrients resulting in better plant growth and yield (Amerian and Stewart, 2001; Wu and Zou, 2009; Bolandnazar et al., 2007; Nedorost et al., 2014). Mycorrhizae can also help absorb certain nutrients more efficiently than plants can and make them more readily available for the plant. With increased moisture and nutrient absorption, plants can become more drought-tolerant. Mycorrhizae also help plants to withstand saline conditions and protect from plant pathogens. A healthy root system can fight soil diseases and weed invasion. Additionally, mycorrhizae increase organic matter content and improve soil structure.
Considering an increasing need for fumigation alternatives to address soilborne pathogens in strawberry, mycorrhizae and other beneficial microbes could be potential tools in maintaining plant health. Additionally, recent studies suggest that entomopathogenic fungi such as Beauveria bassiana, Metarhizium brunneum, and Isaria fumosorosea form mycorrhiza-like and endophytic relationships with various species of plants and could help with plant growth and health (Behie and Bidochka, 2014; Dara et al., 2016). These fungi are currently used for pest management, but their interaction with plants is a new area of research. Understanding this interaction will potentially expand the use of the biopesticides based on these fungi for improving plant growth and health. A study was conducted at Manzanita Berry Farms, Santa Maria in fall-planted strawberry crop during the 2014-2015 production season to evaluate the impact of beneficial microbes on strawberry growth, health, mite infestations, powdery mildew, botrytis fruit rot, and yield.
Methodology:
List of treatments, their application rates and frequencies:
- Untreated control: Received no supplemental treatments other than standard grower practices.
- HealthySoil: NPK (0.1-0.1-0.1).
- BotaniGard ES: Entomopathogenic fungus Beauveria bassiana strain GHA. Rate - 1 qrt in 50 gal for a 30 min transplant dip and 1 qrt/ac every 15 days until January and once a month thereafter until April, 2015.
- Met52: Entomopathogenic fungus Metarhizium brunneum strain F52. Rate – 16 fl oz in 50 gal for a 30 min transplant dip and 16 fl oz/ac every 15 days until January and once a month thereafter until April, 2015.
- NoFly: Entomopathogenic fungus Isaria fumosorosea strain FE9901. Rate – 11.55 oz in 50 gal for a 30 min transplant dip and 11.55 oz/ac every 15 days until January and once a month thereafter until April, 2015.
- Actinovate AG: Beneficial soilborne bacterium Streptomyces lydicus WYEC 108. Rate – 6 oz in 50 gal for a 30 mintransplant dip and 6 oz/ac every month.
- TerraClean 5.0: Hydrogen dioxide and peroxyacetic acid. Rate – 1:256 dilution for a 1 min root dip followed by 2 gal/ac 10 days after planting and then 2 and 1 gal/ac alternated every 15 days until April, 2015.
- TerraGrow: Humic acids, amino acids, sea kelp, glucose based carriers, bacteria – Bacillus licheniformis, B. subtilis, B. pumilus, B. amyloliquefaciens, and B. magaterium, and mycorrhizae – Trichoderma harzianum and T. reesei. Rate – 1.13 g in 10 gal for a 1 min root dip followed by 1.5 lb/ac 10 days after planting and once every month until April, 2015.
- TerraCelan and TerraGrow: Same as individual treatments at the time of planting, but TerraClean at 2 gal/ac and TerraGrow at 1.5 lb/ac 10 days after planting followed by monthly treatments until April, 2015.
- O-MEGA: NPK (0.2-1.0-0.5), bacteria – Azotobacter chroococcum, Azospirillum lipoferum, Lactobacillus acidophilus, Pseudomonas fluorescens, Cellulomonas cellulans and the fungus Aspergillus niger. Rate – 20 ml in 1 gal sprinkled on transplants 30 min before planting followed by 1 qrt/ac every week rest of the season.
Strawberry transplants (variety BG-6.3024) were treated at the time of planting on 6 November, 2014 and treatments are also administered periodically through the drip irrigation system following the abovementioned schedule. Each treatment had two 330' long beds each with four rows of plants. Treatments were randomly arranged in two blocks and two sampling plots (20' long) were established within each bed in a block. The impact of the treatments on plant growth (canopy size), health, spider mite populations, botrytis and powdery mildew severity, and yield were monitored periodically. Plant growth was determined by measuring the canopy size. Plant health was rated on a scale of 0 to 5 where 0=dead, 1=weak, 2=moderate low, 3=moderate high, 4=good, and 5=very good. Powdery mildew severity was determined by observing leaf samples under microscope and rating the severity on a scale of 0 to 4 where 0=no infection, 1=1-25%, 2=26-50%, 3=51-75%, and 4=76-100% of leaf area with powdery mildew. Twenty plants or leaf samples per plot were used for these observations. To monitor botrytis fruit rot, a box of fruits from each plot were held at room temperature and disease was rated 3 and 5 days after harvest on a scale of 0 to 4 where 0=no infection, 1=1-25%, 2=26-50%, 3=51-75%, and 4=76-100% of fruit with botrytis. Yield data were also collected from the plots throughout the production season using grower's harvesting schedule. Mite counts were also taken periodically.
Data were analyzed using analysis of variance and significant means were separated using Tukey's HSD means separation test.
Treating the transplants with different treatment materials and planting in respective beds
Newly transplanted experimental plots.
Chris Martinez (center, front row) and rest of the field crew at Manzanita Berry Farms
Results:
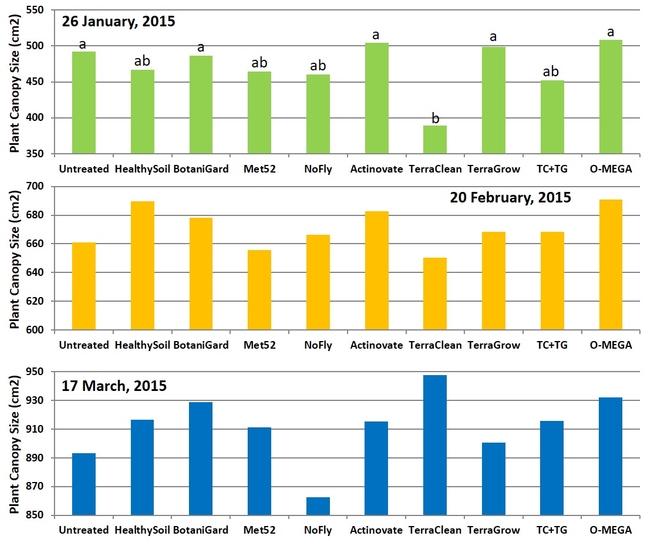
Canopy size: Significant differences (P = 0.002) among treatments were seen only on the first observation date on 26 January, 2015 where TerraClean-treated plants were smaller than some of the treatments. There were no significant differences (P > 0.05) in treatments on the following observations in February and March, however TerraClean-treated plants recovered and plants were larger in some of the treatments.
Size of the plant canopy on three observation dates.
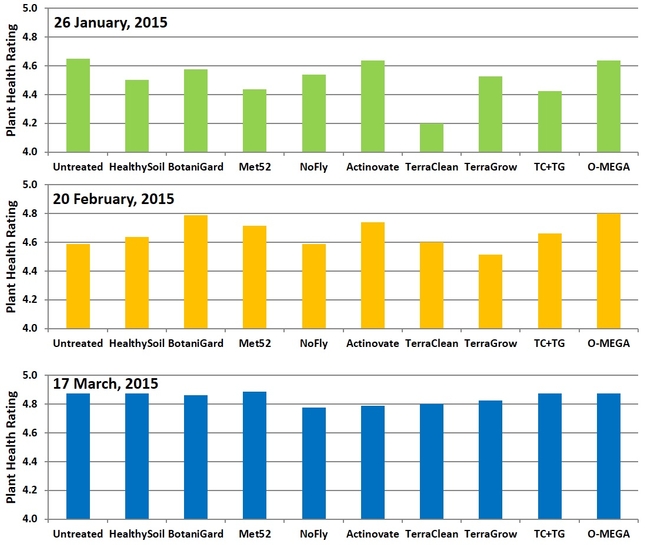
Plant health: Treatments did not have a significant (P > 0.05) impact on plant health. Health ratings varied from 4.2 for TerraClean to 4.6 for untreated, BotaniGard, Actinovate, and O-Mega treatments in January. In February, TerraGrow-treated plants had 4.5 rating and BotaniGard and O-Mega treatments had 4.8. March ratings varied between 4.8 and 4.9 in all the treatments. As there were no soilborne diseases during the study period, the impact of the treatments could not be determined, which was the main objective of the study.
Plant health ratings on three observation dates.
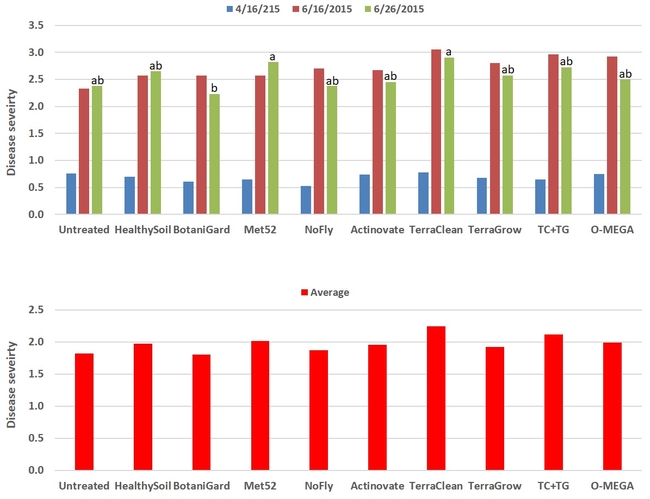
Powdery mildew: Disease severity did not differ among treatments (P > 0.05) on 16 April and 16 June, but significant (P = 0.008) differences were observed on 26 June where BotaniGard-treated plants had the lowest. When data were compared for the three observation dates, severity rating varied from 1.8 for BotaniGard to 2.24 for TerraClean.
Powdery mildew severity on individual observation dates (top) and combined for three observations (bottom)
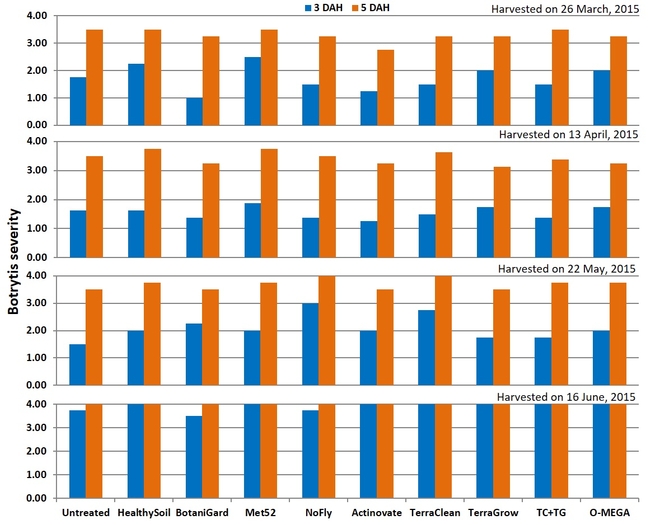
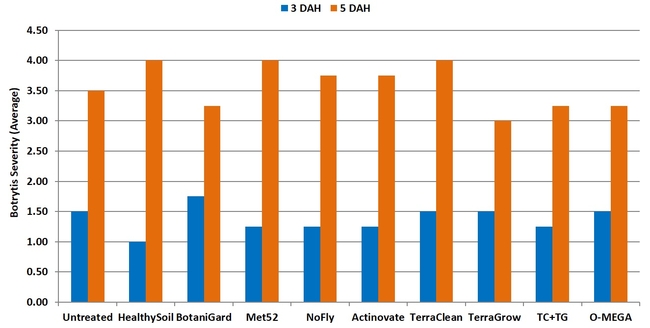
Botrytis fruit rot: There were no significant (P > 0.05) differences among treatments on any of the four observation dates or when data were combined for all observations. In general, fruit rot was less severe 3 days after harvest than 5 days after during the first three observation dates. When data were combined for the observation dates, HealthySoil treatment had a rating of 1 followed by Met52, NoFly, Actinovate, and TerraClean+TerraGrow with a 1.3 rating for 3 days after harvest.
Severity of botrytis fruit rot 3 and 5 days after harvest on individual observation dates (above) and when data were combined (below).
Spider mites: Mite populations were very low in all the plots during observation period and data were not included.
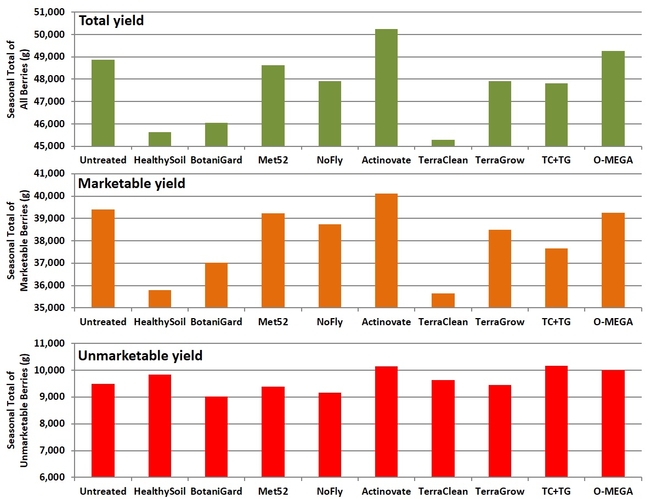
Fruit yield: While the seasonal yield of total, marketable, or unmarketable berries was not significantly (P > 0.05) different for any of the treatments marketable yields had a wider range than unmarketable yields among treatments. The lowest marketable fruit yield was seen in TerraClean (35.6 kg or 79.4 lb) and HealthySoil (35.8 kg or 79.8 lb) while the highest yield was seen in Actinovate (40.1 kg or 89.4 lb) followed by untreated control (39.4 kg or 87.9 lb), O-Mega (39.3 kg or 87.6 lb), Met52 (39.2 kg or 87.4 lb), and NoFly (38.7 kg or 86.3 lb) treatments.
Seasonal yields of total, marketable, and unmarketable strawberries per plot.
This is the first field study evaluating the impact of three popular entomopathogenic fungi along with multiple beneficial microbes on strawberry plant growth, foliar and fruit diseases, and yield. While differences among treatments were not pronounced, it appeared that some had a positive impact on some of the parameters measured. It is interesting to note that yields were higher (although not statistically significant) than the grower standard, HealthySoil. Compared to the grower standard, marketable yield was higher in many other treatments. Since an untreated situation is not common in a commercial field, using beneficial microbes can be useful. Although previous field studies evaluated the impact of with the entomopathogenic fungus B. bassiana in strawberries (Dara, 2013; Dara, 2016), a positive impact on plant growth or yield by I. fumosorosea and M. brunneum in commercial strawberries has never been reported earlier.
Additional studies with different application rates would be useful to understand how beneficial microbes could be exploited more.
Acknowledgments: Thanks to Dave Peck, Manzanita Berry Farms for the collaboration and industry partners for the financial support. Thanks to Chris Martinez and rest of the field crew at Manzanita Berry Farms and Fritz Light and Tamas Zold for the technical assistance.
http://ucanr.edu/articlefeedback
References
Amerian, M.R., and W.S. Stewart. 2001. Effect of two species of arbuscular mycorrhizal fungi on growth, assimilation and leaf water relations in maize (Zea mays). Aspects of Appl. Biol. 63: 1-6.
Behie, S.W., and M.J. Bidochka. 2014. Nutrient transfer in plant-fungal symbioses. Trends in Plant Sci. 19: 734-740.
Bolandnazar, S., N. Aliasgarzad, M.R. Neishabury, and N. Chaparzadeh. 2007. Mycorrhizal colonization improves onion (Allium cepa L.) yield and water use efficiency under water deficit condition. Sci. Horticulturae 114: 11-15.
Dara, S. K. 2013. Entomopathogenic fungus Beauveria bassiana promotes strawberry plant growth and health. UCANR eJournal Strawberries and Vegetables, 30 September, 2013. (http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=11624)
Dara, S. K. 2016. First field study evaluating the impact of the entomopathogenic fungus Beauveria bassiana on strawberry plant growth and yield. UCANR eJournal Strawberrries and Vegetables, 7 November, 2016. (http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=22546)
Dara, S. K., S.S.R. Dara, and S. S. Dara. 2016. First report of entomopathogenic fungi, Beauveria bassiana, Isaria fumosorosea, and Metarhizium brunneum promoting the growth and health of cabbage plants growing under water stress. UCANR eJournal Strawberries and Vegetables, 16 September, 2016.(http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=22131)
Nedorost, L., J. Vojtiskova, and R. Pokluda. 2014. Influence of watering regime and mycorrhizal inoculation on growth and nutrient uptake of pepper (Capsicum annuum L.). VII International symposium on irrigation of horticultural crops, Braun P., M. Stoll, and J. Zinkernagel (eds). Acta Horticulturae 1038:559-564.
Wu, Q.S., and Y. Zou. 2009. Mycorrhizal influence on nutrient uptake of citrus exposed to drought stress. Philippine Agri. Scientist 92: 33-38.
- Author: Surendra K. Dara
A variety of arthropod pests attack strawberries in California and farmers primarily use chemical pesticides for pest management (CDPR, 2014 and Zalom et al. 2014). Recent field studies demonstrated the potential of entomopathogenic fungi, Beauveria bassiana and Metarhizium brunneum in managing important pests such as western tarnished bug, Lygus hesperus in strawberries (Dara 2013a;2014;2015). Entomopathogenic fungi are commonly used as biopesticides where fungal spores cause infections when they come in contact with the target pests. However, these fungi are also reported to endophytically colonize plants (Dara et al., 2013; Behie et al., 2015). Endophytic colonization of B. bassiana in various host plants and the impact on herbivore populations was previously described in some studies (Akello, 2008, Bing and Lewis, 1991, Posada et al., 2007, Tefera and Vidal, 2009, Wagner and Lewis, 2000). An earlier study showed that B. bassiana endophytically colonized strawberry roots, petioles, leaf lamina, pedicels, sepals, and calyxes and persisted up to 9 weeks through soil inoculation (Dara et al., 2013), but its impact on herbivore infestations, especially those with piercing and sucking mouthparts is unknown. A greenhouse study was conducted using green peach aphid, Myzus persicae Sulzer, a minor pest of strawberries, as a model insect to evaluate the impact of endophytic B. bassiana.
Materials and Methods
The study was conducted in a greenhouse using the following treatments: i) untreated control, ii) six weekly soil applications of B. bassiana starting from one week after planting, iii) four weekly foliar applications of B. bassiana starting two weeks after planting, and iv) both soil and foliar applications at respective intervals used with individual applications. Each treatment had four strawberry transplants, obtained from a commercial source and planted in 1 gallon pots (18 cm diameter and 18 cm height) with potting medium composed of a mixture of steam sterilized field soil and perlite. Five grams of Osmocote(R) Slow Release Fertilizer 14-14-14 (Carolina Biological Supply Company, Burlington, NC) was added to each pot followed by watering to the point of saturation. One week after planting, each strawberry plant was infested with 10 pre-adult M. persicae obtained from a greenhouse colony.
Green peach aphids on a potted strawberry plant
For soil treatment of B. bassiana, 1 ml of Mycotrol-O in 100 ml of water was placed around the base of the plant a week after planting and one week prior to aphid infestation. For foliar treatment, 0.25 ml of Mycotrol-O in 100 ml water was sprayed, starting one week after aphid infestation, using a plastic spray bottle until the foliage was thoroughly covered. A polystyrene plate with a hole in the center and a slit across the radius was placed around the base of each plant before administering treatments to avoid cross contamination of soil and foliar treatments. The hole around the plant base was plugged with a ball of cotton.
The number of live and dead aphids, fully expanded leaves, and flower shoots were monitored weekly for a total of seven weeks after artificial infestation and the means for the observation period were calculated. Data were analyzed using ANOVA and significant means were separated using Fisher's Least Significant Difference test. Proportion of live and dead aphids was analyzed after arcsine transformation. Since endophytic colonization of strawberry by B. bassiana was previously reported (Dara et al, 2014), plant tissue was not tested again for the presence of fungus. During the experimental period, average minimum and maximum temperatures were 15.6 and 26.7oC and relative humidity values were 51 and 93%, respectively.
Results
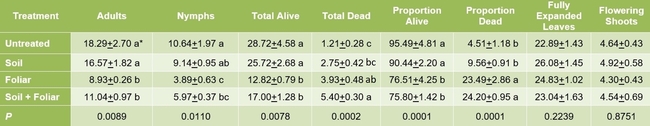
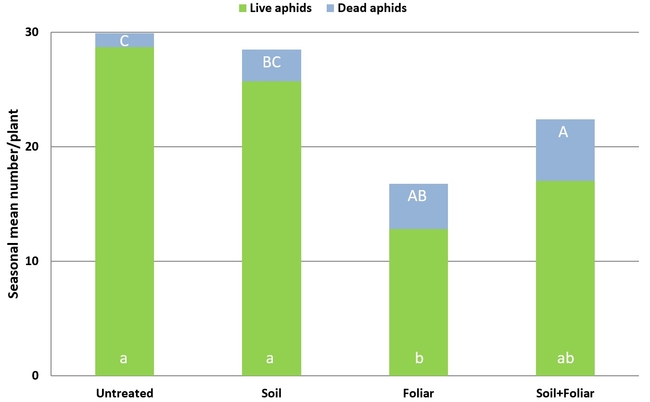
Results indicated that B. bassiana contributed to the mortality of M. persicae through both endophytic and pathogenic modes of action. A significantly higher number of dead aphids was seen on treated plants compared to untreated plants (P = 0.0002). The combination of soil and foliar applications had an additive effect with significantly higher number of dead aphids than soil or foliar applications alone. There was no significant difference in the number (P = 0.0078) or proportion (P = 0.0001) of live aphids or the number of adult aphids (P = 0.0089) between untreated plants and those treated with soil application of B. bassiana. However, there were significantly fewer live aphids where B. bassiana was applied as a foliar spray and a combination of soil application and foliar spray. The impact of treatments on live nymphs was more pronounced with a wider range of significant differences than on live adults. The number of fully expanded leaves and flowering shoots was similar among the treatments (P > 0.05) during the observation period.
* Means followed by the same or no letter within each column are not significantly different at the respective P value in the bottom
Impact of soil and foliar applications of B. bassiana on green peach aphid numbers and strawberry plant
Impact of soil and foliar applications of B. bassiana on green peach aphids on strawberry plants
Discussion
Although entomopathogenic fungi are known to have endophytic interactions with various plant species, how this interaction influences herbivore populations is not fully understood. Several studies shed some light on this new area of research, but they primarily include insects with chewing mouthparts such as the banana weevil, Cosmopolites sorditus on banana (Akello et al., 2008), the corn ear worm, Helicoverpa zea on tomato (Powell et al., 2009), and the European corn borer, Ostrinia nubilalis on corn (Bing and Lewis, 1991, Lewis et al., 1996) except for a recent report of endophytic B. bassiana and Purpureocilium licacinum impacting the survival and reproduction of cotton aphid, Aphis gossypii Glover on cotton (Castillo Lopez et al., 2014). Antibiosis is thought to be one of the mechanisms for the endophytic entomopathogens to affect herbivores (Castillo Lopez et al., 204, Vega et al., 2008).
The current study clearly indicated that B. bassiana affected the mortality of M. persicae as an endophyte and an entomopathogen. Having an additive effect through endophytic interaction as well as infection is useful for increasing pest control efficacy in practical agriculture. Entomopathogenic fungi and other microbial control agents are generally perceived to be less effective than chemical pesticides and improved efficacy through multiple modes of action adds value to microbial control. In an earlier study, greenhouse strawberry plants that received soil application of M. brunneum withstood infestations of twospotted spider mite, Tetranychus urticae Koch, better than untreated plants (Dara and Dara 2015). Endophytic colonization of the fungus could not be determined by surface sterilizing and plating the plant tissue on selective medium, but treated plants performed better than control plants under mite pressure indicating a positive impact of M. brunneum on strawberry plants.
In the current study, while the mortality of aphids was higher with the combined treatment of soil and foliar applications, surviving aphids did not follow the same trend showing slightly higher numbers wherever soil applications were made. In general, plants that received soil application of B. bassiana appeared to be healthier than untreated or foliar treatment alone and although not significantly different, plants that received the soil treatment had a slightly higher number of leaves during the observation period possibly contributing to higher surviving aphids. Other studies conducted in California also support this idea that entomopathogenic fungi, including B. bassiana, promote plant growth (Dara, 2013b, Dara et al. 2014).
This is the first report of the impact of endophytic B. bassiana on the mortality of M. persicae on strawberry laying foundation for additional studies with major pests such as L. hesperus. Entomopathogenic fungi can play a significant role in integrated pest management and studies that elucidate their interaction with plants and pests will help promote their use in sustainable agriculture.
Acknowledgements
Thanks to Jaclyn Wiley and Melody Carter for their technical assistance and David Headrick, Cal Poly for providing aphids and the greenhouse space for the study.
References
Akello, J., Dubois, T., Coyne, D., Kyamanywa, S. 2008. Endophytic Beauveria bassiana in banan (Musa spp.) reduces banana weevil (Cosmopolites sordidus) fitness and damage. Crop Protection 27: 1437-1441.
Behie, S. W., Jones, S. J., Bidochka, M. J. 2015. Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecology 13: 112-114.
Bing, L. A., Lewis, L. C. 1991. Suppression of Ostrinia nubilalis (Hubner) (Lepidoptera: Pyraliade) by entomopathogenic Beauveria bassiana(Balsamo) Vuillemin. Environ. Entomol. 20, 1207-1211.
California Department of Pesticide Regulation (CDPR). 2014. Summary of pesticide use report data 2012: Indexed by commodity.
Dara, S. K. 2013a. Strawberry IPM study 2013: managing insect pests with chemical, botanical, and microbial pesticides. http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=19290.
Dara, S. K. 2013b. Entomopathogenic fungus Beauveria bassiana promotes strawberry plant growth and health. http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=11624.
Dara, S. K. 2014. Strawberry IPM study 2014: managing insect pests with chemical, botanical, microbial, and other pesticides. http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=19294.
Dara, S. K. 2015. Strawberry IPM study 2015: managing insect pests with chemical, botanical, microbial, and mechanical control options. http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=19641.
Dara, S. K., Dara, S. R, Dara, S. S. 2013. Endophytic colonization and pest management potential of Beauveria bassiana in strawberries. J. Berry Res. 3: 203-211.
Dara, S. K., Dara, S. S., Dara, S. S. 2014. Entomopathogenic fungi as plant growth enhancers. 47th Annual Meeting of the Society for Invertebrate Pathology and International Congress on Invertebrate Pathology and Microbial Control, August 3-7, Mainz, Germany pp. 103-104.
Lewis, L. C., Berry, E. C., Obrycki, J. J., Bing, L. A. 1996. Aptness of insecticides (Bacillus thuringiensis and carbofuran) with endophytic Beauveria bassiana, in suppressing larval populations of the European corn borer. Agri. Eco. Environ. 57, 27-34.
Posada, F., Aime, M. C., Peterson, S. W., Aehner, S. A., Vega, F. E. 2007. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana(Ascomycota: Hypocreales). Mycol. Res. 111: 748-757.
Tefera, T., Vidal, S. 2009. Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. BioCon. 54: 663-669.
Vega, F. E., Posada, F., Aime, M. C., Pava-Ripoll, M., Infante, F., Rehner, S. A. 2008. Entomopathogenic fungal endophytes. Biol. Con. 46: 72-82.
Wagner, B. L., Lewis, L. C. 2000. Colonization of corn, Zea mays, by the entomopathogenic fungus Beauveria bassiana. Appl. Environ. Microbiol. 2000: 3468-3473.
Zalom, F. G., Bolda, M. P., Dara, S. K., Joseph, S. 2014. UC IPM Pest Management Guidelines: Strawberry. University of California Statewide Integrated Pest Management Program. Oakland: UC ANR Publication 3468. June, 2014.
- Author: Surendra Dara
The combination of 1,3 dichloropropene (1,3-D)and cholorpicrin is a popular choice for fumigating strawberry fields after methyl bromide. It is very effective and convenient to administer through the drip irrigation system. However, 1,3-D can result in phytotoxicity if transplanting takes place before the fumigant completely dissipates.
Phytotoxicity from 1,3-D
Symptoms of phytotoxicity from 1,3-D include yellowing of leaves purple coloration and stunted plant growth. Newly emerged leaves will look normal and plants appear to recover to some extent, but subsequent growth and yield potential can be affected.

Strawberry plants (variety Albion) about 85 days after transplanting (above and below). Stunted plant growth, discoloration of the foliage with purple tinge indicated injury from 1,3-D. Transplanting took place 16 days after fumigation. (Photos by Surendra Dara)


Purple coloration on older leaves can still be seen even up to 110 days after transplanting as a result of 1,3-D injury (Photo by Surendra Dara)

A typical plant from 1,3-D injured field 110 days after transplanting (left) is smaller than a typical plant from a neighboring field which was transplanted about 3 weeks later and not subjected to 1,3-D injury (right) (Photos by Surendra Dara)
Avoiding fumigant injury
Proper planting interval after fumigation is very important to avoid phytotoxicity and subsequent crop losses. It usually takes two or more weeks for the fumigant to dissipate depending on the type of soil, method of application, plastic mulch, and soil temperature and moisture conditions. Use of virtually impermeable film (VIF) or totally impermeable film (VIF) increase the effectiveness of fumigation, but retain the fumigant for a longer period in the soil requiring additional time before planting. Chloropicrin and 1,3-D usually are ideal for dryer soils and require longer planting interval in moist soils. In drip fumigation, adequate amount of water to apply fumigants is important. Excessive water can dilute the fumigant concentration and reduce its effectiveness. Insufficient water limits the distribution and may increase the volatilization of the fumigant and thus reduces its effectiveness. A minimum of three weeks of plant-back time is recommended for drip applied 1,3-D products. It is important to refer to the product label for proper fumigation procedures and planting interval. A healthy start is essential for the season long performance of the strawberry plant and realizing the maximum yield potential.
http://ucanr.edu/articlefeedback
References
2008. IPM for strawberries. UCANR publication 3351.
Santos, B. M. 2007. Life after methyl bromide: research on 1,3-dichloropropene plus chloropicrin in Florida. University of Florida Cooperative Extension Service publication HS1119.
- Author: Surendra Dara
Lygus bug (Lygus spp.) is a major strawberry pest in California affecting the yield and quality of berries. Although strawberry is not a preferred host for lygus bug compared to other hosts like alfalfa, as the strawberry crop is available almost throughout the year, it seems to serve as a permanent habitat for this pest. Flowering weed hosts like wild radish and wild mustard also harbor lygus bug populations.
Regular monitoring to make treatment decisions, managing weeds or alternative hosts to limit the spread of lygus bug to strawberry fields, using flowering hosts as trap crops, conserving natural enemies, vacuuming, and chemical control are common techniques used for lygus bug management. Calculating degree-days is an approach that can help estimate when immature or adult stages of lygus bug will be seen in the fields so that appropriate treatment decision can be made.
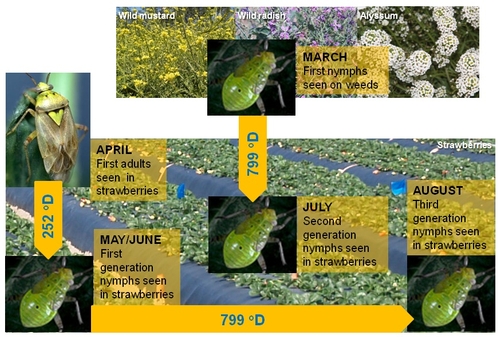
Lygus degree-day model for strawberries (Photo illustration by Surendra Dara)
Degree-day is the unit of measurement for physiological time for insect maturity. In other words, it is the time an insect takes from one point to another point in its life cycle depending on the temperature. The amount of heat accumulated in 24 hours when the temperature is one degree above the lower developmental threshold is a degree-day (oD). The lower developmental threshold for lygus bug is 54 oF and it requires 252 oD (in Fahrenheit) for egg stage and 371 oD for nymphal stages to complete. Adults require 176 oD before they start laying eggs. So, it takes a total of 799 oD from egg to next generation egg. By monitoring the presence of lygus bug and daily temperatures, degree-days can be calculated to make treatment decisions against nymphal stages following UC IPM guidelines.
For example, if lygus nymphs are first seen in March on weed hosts or adults are first seen in strawberries in April, degree-day model predicts that first generation nymphs from adults can be seen in strawberries around May-June after accumulating 252 oD. Second generation nymphs, from the nymphs on weeds, can be seen in July in strawberries after accumulating 799 oD. The third generation nymphs from first generation can also be seen after 799 oD in August.
California Strawberry Commission, in collaboration with growers in the Watsonville and the Santa Maria areas, is working on monitoring lygus populations and calculating degree days. Data loggers that record temperatures and calculate degree-days were recently set up in about 35 strawberry fields in these two areas. Growers periodically monitor the fields and once the adult lygus bug is found, calculation will begin until 252 oD are accumulated. This indicates the time when the first generation nymphs emerge so that appropriate management decisions can be made. Although lygus bugs can move into the strawberry fields and deposit eggs several times during the growing season, resulting in the emergence of nymphs at different times, degree-day model can still help tackling the first generation nymphs.

Andrew Kramer from California Strawberry Commission explaining the functioning of the unit to Dave Peck, Manzanita Farms, Santa Maria

One degree-day (01 at the extreme right) was accumulated one day after installing the data logger in one of the Santa Maria fields
Visit UC IPM web resources to find out more information about degree-days (www.ipm.ucdavis.edu/PHENOLOGY/ma-lygus_bug.html) or to calculate degree-days by uploading your own temperature data (http://www.ipm.ucdavis.edu/WEATHER/ddretrieve.html).