- Author: Surendra K. Dara
Mechanisms of insecticide resistance in insects.
Use of biopesticides or non-chemical pesticides is encouraged as a part of integrated pest management (IPM) for environmental and human safety and to reduce the risk of insecticide resistance. With the increase in biopesticide use in both organic and conventional cropping systems, it is a good time to review the potential of insect resistance to botanical and microbial pesticides.
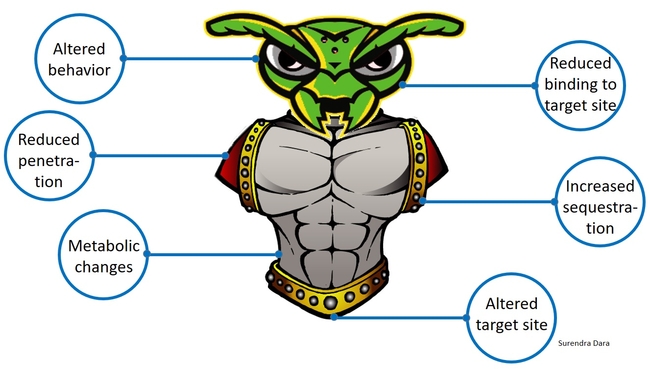
Insects and mites develop resistance to chemical pesticides through genetic, metabolic, or behavioral changes resulting in reduced penetration of toxin, increased sequestration or excretion, reduced binding to the target site, altered target site that prevents binding of the toxin, or reduced exposure to the toxin through modified behavior. When the active ingredient is a toxic molecule and has the mode of action similar to that of a chemical compound, regardless of the plant or microbial origin, arthropods are more likely to develop resistance through one or more of the abovementioned mechanisms. When the mode of action is infection by a microorganism, rather than a toxin, arthropods are less likely to develop resistance. Under natural circumstances, plants, insects, natural enemies, and beneficial or harmful microbes continuously co-evolve and adapt to changing environment. When there is a higher selection pressure, such as indiscriminate use of chemical pesticides, increased mutagenesis can lead to resistance issues. A good understanding of insect resistance to biopesticides will help minimize potential risks and improve their efficient use in IPM.
Resistance to botanical pesticides
Nicotine, an alkaloid from Nicotiana spp., is one of the earlier botanical pesticides known. Although nicotine is not currently used as an insecticide, its synthetic alternatives – neonecotinoids – are commonly used against several pests. Botanical insecticide pyrethrum, extracted from the flowers of Chrysanthemum cinerariaefolium, contains insecticidal pyrethrins (synthetic pyrethrins are referred to as pyrethroids). Although insect resistance to pyrethrum or pyrethroid compounds has been known (Whitehead, 1959; Immaraju et al., 1992; Glenn et al., 1994), they have been effectively used against a number of pests through careful placement in IPM, organic, or conventional management strategies. Additionally, pyrethrin products have been effectively used along with piperonyl butoxide, which acts as a synergist and resistance breaker (Gunning et al. 2015).
Another botanical insecticidal compound, azadirachtin, is a tetranortriterpenoid limonoid from neem (Azadirachta indica) seeds, which acts as an insecticide, antifeedant, repellent and insect growth regulator. While neem oil, which has a lower concentration of azadirachtin, has been used in the United States as a fungicide, acaricide, and insecticide for a long time, several azadirachtin formulations in powder and liquid forms have become popular in recent years and were found effective in managing important pests (Dara 2015a and 2016). Feng and Isman (1995) reported that the green peach aphid, Myzus persicae developed resistance to pure azadirachtin under artificially induced selection pressure after 40 generations, but did not develop resistance to a refined neem seed extract. They suggested that natural blend of azadirachtin compounds in a biopesticide would not exert selection pressure that could lead to resistance. Additionally, Mordue and Nisbet (2000) discussed that azadirachtin can play a role in insecticide resistance management because it reduces the detoxification enzyme production as a protein synthesis inhibitor. Azadirachtin also improved the efficacy of other biopesticides in multiple studies (Trisyono and Whalon, 2000; Dara, 2013 and 2015b).
Insects feeding on plant allelochemicals can develop cross-resistance to insecticides (Després et al., 2007). For example, overproduction of detoxification enzymes such as glutathione S-transferases and monooxygenases in the fall armyworm, Spodoptera frugiperda,when it fed on corn and cowpea, respectively, imparted cross-resistance to various chemical pesticides. It is important to keep this in mind when botanical pesticides are used to detect potential resistance issues.
Resistance to bacterial biopesticides
Bacillus thuringiensis (Bt)is a gram-positive soil bacterium, which contains crystalline toxic protein that is activated upon ingestion by an insect host, binds to the receptor sites in the midgut, and eventually causes insect death. Since the mode of action involves a toxin rather than bacterial infection, several insects developed resistance to Bt pesticides or transgenic crops that contain Bt toxins (Tabashnik et al., 1990; McGaughey and Whalon, 1992; Tabashnik, 1994; Iqbal et al., 1996). However, Bt pesticides are still very popular and used against a variety of lepidopteran (Bt subsp. aizawai and Bt subsp. kurstaki), dipteran (Bt subsp. israelensis and Bt subsp. sphaericus), and coleopteran (Bt subsp. tenebrionis) pests.
Spinosad is a mixture of macrocyclic lactones, spinosyns A and spinosyns D, derived from Saccharopolyspora spinosa, an actinomycete gram-positive bacterium, and is used against dipteran, hymenopteran, lepidopteran, thysanopteran, and other pests. Spinosad products, while naturally derived are registered as chemical pesticides, not as biopesticides. Insect resistance to spinosad later led to the development of spinetoram, which is a mixture of chemically modified spinosyns J and L. Both spinosad and spinetoram are contact and stomach poisons and act on insect nervous system by continuous activation of nicotinic acetylcholine receptors. However, insect resistance to both spinosad (Sayyed et al., 2004; Bielza et al., 2007) and spinetoram (Ahmad and Gull, 2017) has been reported due to extensive use of these pesticides. Cross-resistance between spinosad and some chemical insecticides has also occurred in some insects (Mota-Sanchez et al., 2006; Afzal and Shad, 2017).
Resistance to viral biopesticides
Baculovirus infections in lepidoptera have been known for centuries, especially in silkworms. Currently, there are several commercial formulations of nucleopolyhedroviruses (NPV) and granuloviruses (GV). When virus particles are ingested by the insect host, usually lepidoptera, they invade the nucleii of midgut, fatbody, or other tissue cells and kill the host. Baculoviruses are generally very specific to their host insect species and can be very effective in bringing down the pest populations. However, variations in the susceptibility of certain insect populations and development of resistant to viruses has occurred in several host species (Siegwart et al., 2015). Resistance to different isolates of Cydia pomonella granulovirus (CpGV-M, CpGV-S) in codling moth (Cydia pomonella) populations is well known in Germany and other parts of Europe (Sauer et al., 2017a & b).
Resistance to fungal biopesticides
There are several fungi that infect insects and mites. Fungal infection starts when fungal spores come in contact with an arthropod host. First, they germinate and gain entry into the body by breaching through the cuticle. Fungus later multiplies, invades the host tissues, kills the host, and emerges from the cadaver to produce more spores. Entomophthoralean fungi such as Entomophthora spp., Pandora spp., and Neozygites spp. can be very effective in pest management through natural epizootics, but cannot be cultured in vitro for commercial scale production. Hypocrealean fungi such as Beauveria bassiana, Isarea fumosorosea, Metarhizium brunneum, and Verticillium lecanii,on the other hand, can be mass-produced in vitro and are commercially available. These fungi are comparable to broad-spectrum insecticides and are pathogenic to a variety of soil, foliar, and fruit pests of several major orders. Since botanical, bacterial, and viral biopesticides have insecticidal metabolites, proteins, or viral particles that have specific target sites and mode of action, insects have a higher chance of developing resistance through one or more mechanisms. Although fungi also have insecticidal proteins such as beauvericin in B. bassiana and I. fumosorosea and dextruxin in M. anisopliae and M. brunneum, their mode of action is more through fungal infection and multiplication and arthropods are less prone to developing resistance to entomopathogenic fungi. However, insects can develop resistance to entomopathogenic fungi through increased melanism, phenoloxidase activity, protease inhibitor production, and antimicrobial and antifungal peptide production (Wilson et al., 2001; Zhao et al., 2012; Dubovskiy et al., 2013). It appears that production of detoxification enzymes in insects against fungal infections can also impart resistance to chemical pesticides. Infection of M. anisopliae in the larvae of greater wax moth, Galleria mellonella, increased dexotification enzyme activity and thus resistance to malathion (Serebrov et al., 2006).
These examples show that insects can develop resistance to biopesticides in a manner somewhat similar to chemical pesticides, but due to the typically more complex and multiple modes of action, at a significantly lesser rate depending on the kind of botanical compound or microorganism involved. Resistance to entomopathogenic fungi is less common than with other entomopathogens. Since biopesticide use is not as widespread as chemical pesticides, the risk of resistance development is less for the former. However, excessive use of any single tool has the potential for resistance or other issues and IPM, which uses a variety of management options, is always a good strategy.
Acknowledgements: Thanks to Pam Marrone for reviewing the manuscript.
References
Afzal, M.B.S. and S. A. Shad. 2017. Spinosad resistance in an invasive cotton mealybug, Phenacoccus solenopsis: cross-resistance, stability and relative fitness. J. Asia-Pacific Entomol. 20: 457-462.
Ahmad M. and S. Gull. 2017. Susceptibility of armyworm Spodoptera litura (Lepidoptera: Noctuidae) to novel insecticides in Pakistan. The Can. Entomol. 149: 649-661.
Bielza, P., V. Quinto, E. Fernández, C. Grávalos, and J. Contreras. 2007. Genetics of spinosad resistance in Frankliniella occidentalis (Thysanoptera: Thripidae). J. Econ. Entomol. 100: 916-920.
Dara, S. K. 2013. Strawberry IPM study 2012: managing insect pests with chemical, botanical, and microbial pesticides. UCANR eJournal Strawberries and Vegetables March 13, 2013 (http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=9595).
Dara, S. K. 2015a. Root aphids and their management in organic celery. CAPCA Adviser 18(5): 65-70.
Dara, S. K. 2015b. Strawberry IPM study 2015: managing insect pests with chemical, botanical, microbial, and mechanical control options. UCANR eJournal Strawberries and Vegetables November 30, 2015 (http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=19641).
Dara, S. K. 2016. Managing strawberry pests with chemical pesticides and non-chemical alternatives. Intl. J. Fruit Sci. https://doi.org/10.1080/15538362.2016.1195311
Després, L., J.-L. David, and C. Gallet. 2007. The evolutionary ecology of insect resistance to plant chemicals. Trends in Ecol. Evol. 22: 298-307.
Dubovskiy, I. M., M.M.A. Whitten, O. N. Yaroslavtseva, C. Greig, V. Y. Kryukov, E. V. Grizanova, K. Mukherjee, A. Vilcinskas, V. V. Glupov, and T. M. Butt. 2013. Can insects develop resistance to insect pathogenic fungi? PloS one 8: e60248.
Feng, R. and M. B. Isman. 1995. Selection for resistance to azadirachtin in the green peach aphid, Myzus persicae. Experientia 51: 831-833.
Glenn, D. C., A. A. Hoffmann, and G. McDonald. 1994. Resistance to pyrethroids in Helicoverpa armigera (Lepidoptera: Noctuidae) from corn: adult resistance, larval resistance, and fitness effects. J. Econ. Entomol. 87: 1165-1171.
Gunning, R., G. Moores, and M. Balfe. 2015. Novel use of pyrethrum to control resistant insect pests on cotton. Acta Hort. 1073: 113-118. https://doi.org/10.17660/ActaHortic.2015.1073.16
Immaraju, J. A., T. D. Paine, J. A. Bethke, K. L. Robb, and J. P Newman. 1992. Western flower thrips (Thysanoptera: Thripidae) resistance to insecticides in coastal California greenhouses. J. Econ. Entomol. 85: 9-14.
Iqbal, K., R.H.J. Vekerk, M. J. Furlong, P. C. Ong, S. A. Rahman, and D. J. Wright. 1996. Evidence for resistance to Bacillus thuringiensis (Bt) subsp. kurstaki HD-1, Bt subsp. aizawai and Abamectin in field populations of Plutella xylostella from Malaysia. Pest Manag. Sci. 48: 89-97.
McGaughey, W. H. and M. E. Whalon. 1992. Managing insect resistance to Bacillus thuringiensis toxins. Science 258: 1451-1455.
Mordue, A. J. and A. J. Nisbet. 2000. Azadirachtin from the neem tree Azadirachta indica: its action against insects. An. Soc. Entomol. Bras. 29: 615-632.
Mota-Sanchez, D., R. M. Hollingworth, E. J. Grafius, and D. D. Moyer. 2006. Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Pest Manag. Sci. 62: 30-37.
Sauer, A. J., E. Fritsch, K. Undorf-Spahn, P. Nguyen, F. Marec, D. G. Heckel, and J. A. Jehle. 2017a. Novel resistance to Cydia pomonella granulovirus (CpGV) in codling moth shows autosomal and dominant inheritance and confers cross-resistance to different CpGV genome groups. PLoS ONE 12(6): e0179157.
Sauer, A. J., S. Schulze-Bopp, E. Fritsch, K. Undorf-Spahn, and J. A. Jehle. 2017b. A third type of resistance of codling moth against Cydia pomonella granulovirus (CpGV) shows a mixture of a Z-linked and autosomal inheritance pattern. Appl. Environ. Microbiol. AEM-01036.
Sayyed, A. H., D. Omar, and D. J. Wright. 2004. Genetics of spinosad resistance in a multi-resistant field-selected population of Plutella xylostella. Pest Manag. Sci. 60: 827-832.
Serebrov, V. V., O. N. Gerber, A. A. Malyarchuk, V. V. Martemyanov, A. A. Alekseev, and V. V. Glupov. Effect of entomopathogenic fungi on detoxification enzyme activity in greater wax moth Galleria mellonella L. (Lepidoptera, Pyralidae) and role of detoxification enzymes in development of insect resistance to entomopathogenic fungi. Anim. Human Physiol. 33: 581-586.
Siegwart, M., B. Graillot, C. B. Lopez, S. Besse, M. Bardin, P. C. Nicot, and M. Lopez-Ferber. 2015. Resistance to bio-insecticides or how to enhance their sustainability: a review. Front. Plant Sci. 6:381. https://doi.org/10.3389/fpls.2015.00381
Tabashnik, B. 1994. Evolution of resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 39: 47-79.
Tabashnik, B. E., N. L. Cushing, N. Finson, and M. W. Johnson. 1990. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 83: 1671-1676.
Trisyono, A. and M. E. Whalon. 2000. Toxicity of neem applied alone and in combination with Bacillus thuringiensis to Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 92: 1281-1288.
Whitehead, G. B. 1959. Pyrethrum resistance conferred by resistance to DDT in the blue tick. Nature 184: 378-379.
Wilson K., S. C. Cotter, A. F. Reeson, and J. K. Pell. 2003. Melanism and disease resistance in insects. Ecology Letters 4: 637-649.
Zhao, P., Z. Dong, J. Duan, G. Wang, L. Wang, Y. Li, Z. Xiang, and Q. Xia. 2012. Genome-wide identification and immune response analysis of serine protease inhibitor genes in the silkworm, Bombyx mori. PloS one 7: e31168.
- Author: Surendra K. Dara
Strawberry is an important commodity in California with a crop value of $2 billion (NASS, 2013). Lygus bug or western tarnished plant bug (Lygus hesperus), twospotted spider mite (Tetranychus urticae), greenhouse whitefly (Trialeurodes vaporariorum), and western flower thrips (Frankliniella occidentalis) are considered as important arthropod pests of strawberries which can cause significant yield losses. According to the Pesticide Use Report of California Department of Pesticide Regulation (2014), more than 200,000 lb of chemical insecticide and miticide active ingredients were used in strawberries in 2012. Among the 50,000 lb of biorational active ingredients that were additionally applied, 97% were Bacillus thuringiensis products used against lepidopteran pests. Apart from the release of various species of predatory mites against twospotted spider mites, pest management in strawberries is mainly dependent on chemical pesticides and IPM is generally limited to the rotation of pesticides in different modes of action groups.
In an effort to develop an effective IPM program with a particular emphasis on lygus bug management, research has been conducted for the past few years in Santa Maria to evaluate the role of various non-chemical alternatives. Field studies in 2013 showed that botanical and microbial pesticides can be effectively used in combination and rotation with chemical pesticides (Dara, 2014). Additional studies were conducted in 2014 to evaluate the efficacy of various combinations and rotations of new and existing chemical pesticides along with botanical, earth-based, and microbial pesticides.
Methodology
A large scale field study was conducted during June and July, 2014 in a conventional strawberry field of variety Del Rey at Goodwin Berry Farms, Santa Maria. Chemical pesticides included those from IRAC mode of action groups 3A (sodium channel modulators) 4A (neonicotinoids), 4C (sulfoximines), 6 (chloride channel activators), 9C (selective homopteran feeding blockers), and 15 (inhibitors of chitin biosynthesis). Additionally, diatomaceous earth, azadirachtin, and two entomopathogenic fungi, Beauveria bassiana and Metarhizium brunneum (formerly known as M. anisopliae) were also used. Diatomaceous earth is a powder form of fossilized remains of diatoms and contains silicon dioxide as an active ingredient. Silicon dioxide interferes with the integrity of the cuticle by absorbing the waxy layer and causes mortality due to desiccation. Both B. bassiana and M. brunneum are soilborne entomopathogenic fungi which cause infection when a conidiospore comes in contact with an insect or a mite. Azadirachtin, a secondary metabolite present in neem seed, is a limonoid compound which interferes with the synthesis of various proteins and thus affects molting, mating, sexual communication, and reproductive ability. It also has insecticidal properties and acts as an antifeedant and repellent. Using these alternatives can help pest management which is sometimes difficult to achieve with chemical pesticides alone.
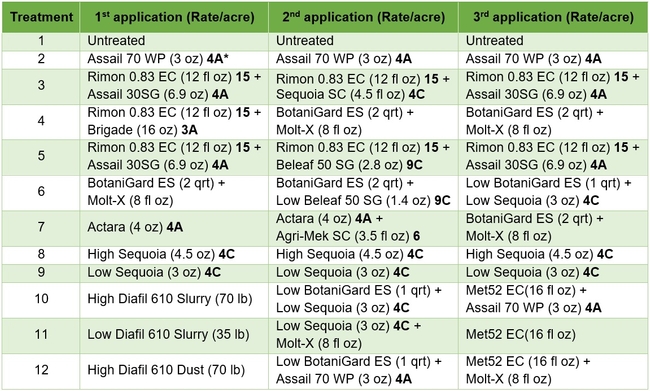
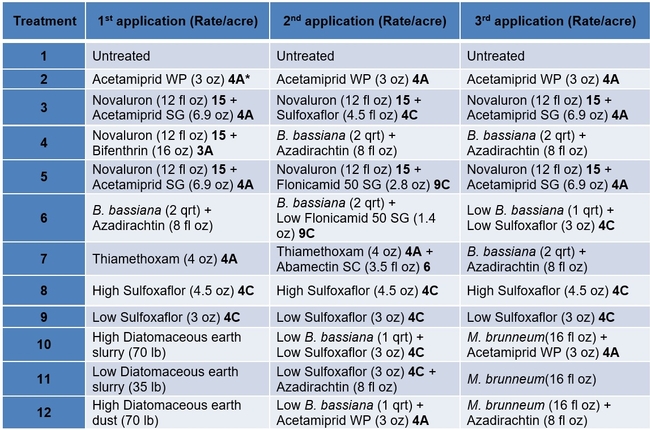
Treatments included an untreated control, a wettable powder formulation of acetamiprid as the grower standard, and other materials in different combinations and rotations (Tables 1 and 2). Each plot had seven 75' long and 64” wide beds and treatments were replicated four times in a randomized complete block design. Treatments were administered late afternoon or in the evening using a tractor-mounted sprayer except for diatomaceous earth dust which was applied by a backpack dust applicator. Three applications were made at 7-8 day intervals and observations were made once before the first application and 5-6 days after each application. On each observation date, 20 plants were randomly sampled from the middle three beds of each plot by gently beating the plant to dislodge insects into a container. The number of aphids, lygus bugs (young and mature nymphs and adults), thrips, whitefly adults, and various species of natural enemies were counted from each plant. Natural enemy complex included bigeyed bug (Geocoris spp.), minute pirate bug (Orius spp.), lacewing (Chrysoperla spp. and Chrysopa spp.), damsel bug (Nabis spp.), lady beetle (multiple species), parasitoids (multiple species), and spiders (multiple species). Data were analyzed using statistical procedures.
Table 1. List of treatments used in this study and their application rates per acre – Active ingredients
*3A Sodium channel modulators 4A Neonecotinoids, 4C Sulfoxamines, 6 Chloride channel activators, 9C Selective homopteran feeding blockers, and 15 Inhibitors of chitin biosynthesis.
Table 2. List of treatments used in this study and their application rates per acre – Trade names
*3A Sodium channel modulators 4A Neonecotinoids, 4C Sulfoxamines, 6 Chloride channel activators, 9C Selective homopteran feeding blockers, and 15 Inhibitors of chitin biosynthesis.
Results and Discussion
Actual numbers of various pests and natural enemies are presented in Table 3 and percent change post-treatment compared to pre-treatment is presented in different figures.
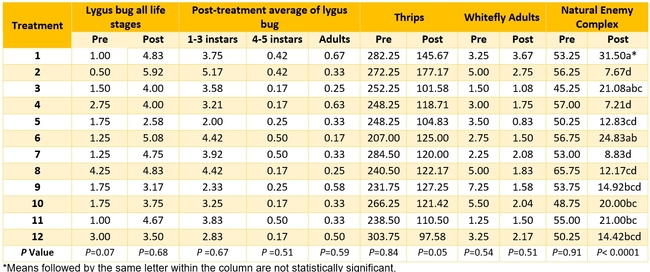
Table 3. Pest and natural enemy populations from various treatments before and after treatment per 20 sample plants. Post-treatment counts include averages for three spray applications. Refer to Tables 1 and 2 for the list of treatments.
Aphid: Negligible number of aphids was seen only in few treatments and data are not presented.
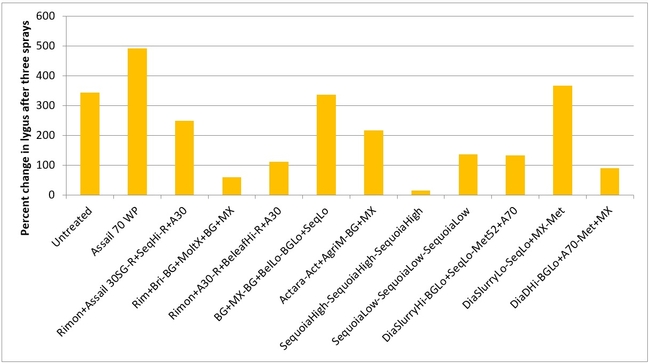
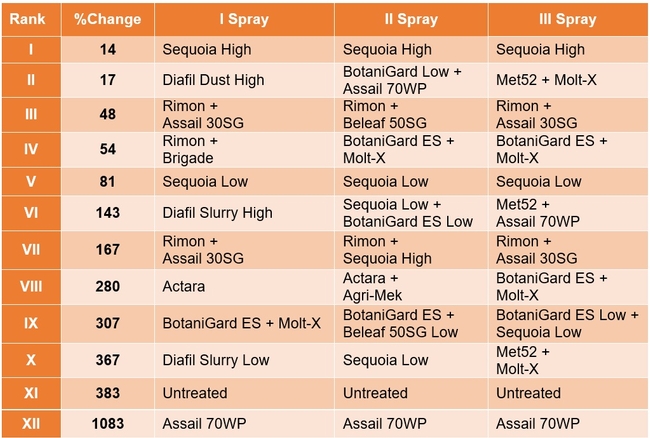
Lygus bug: Lygus numbers increased in all treatments after treatment and there were no statistical differences (P > 0.05). However, when the percent change, compared to pre-treatment counts, was considered, some treatments appeared to be more effective than others in preventing population buildup. The high rate of Sequoia (treatment 8) limited the increase to 14% followed by the rotation of Diafil high rate-BotaniGard low rate+Assail 70 WP-Met 52+Molt-X (treatment 12) indicating the potential of non-chemical alternatives for lygus bug management (Table 4). When BotaniGard+Molt-X combination was applied twice after Rimon+Brigade combination (treatment 4), it appeared to be the fourth best rotation limiting the population build up to 54%. Untreated control and Assail 70 WP had the highest lygus numbers with 383% and 1083% increase, respectively.
Percent change in all stages of lygus bugs after three spray applications.
Treatments ranked according to their efficacy as expressed by the percent change/control of lygus bugs after three spray applications.
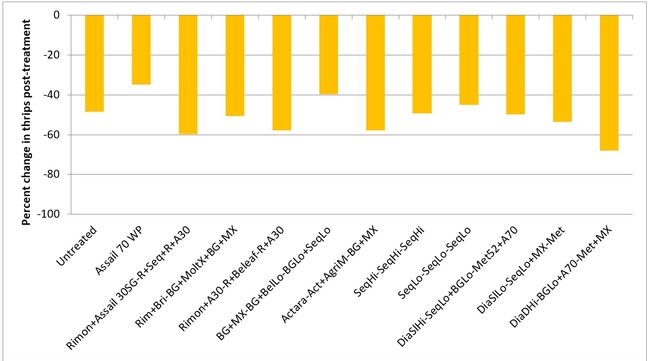
Thrips:There was a general reduction in thrips numbers post-treatment. There was a 48% reduction in their post-treatment numbers in untreated control while it varied from 35% treatment 2 to 68% in treatment 12.
Percent change in western flower thrips populations after three spray applications.
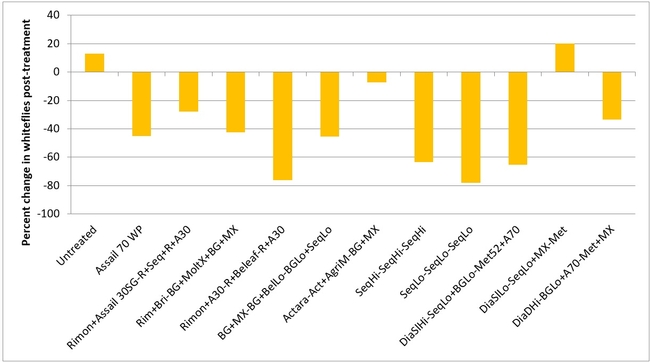
Whitefly adult:Most of the treatments reduced whitefly populations except for one treatment where there was a 20% increase (treatment 11 – Diafil low rate followed by Sequoia low rate+Molt-X, and Met 52) compared to a 13% in untreated control. There was a 7 to 78% reduction in whitefly populations in all other treatments.
Percent change in greenhouse whitefly adult populations after three spray applications.
Natural enemy complex:The number of big-eyed bug, parasitoids, and spiders significantly varied among various treatments post-treatment (P < 0.05, data not shown). When the percent change was considered for the entire natural enemy complex, there was a reduction in all treatments with 41% reduction in untreated control and 53-86% reduction in various treatments.
Diafil application left a white deposit on strawberry plants for several days making the berries unmarketable. It may not be practical for managing lygus bug, which usually appears after fruit production starts.
These results support last year's data in demonstrating the potential of non-chemical alternatives such as microbial and botanical pesticides. These tools are essential for sustainable pest management and can make a significant reduction in chemical pesticide use without compromising the control efficacy.
http://ucanr.edu/articlefeedback
References
California Department of Pesticide Regulation. 2014. Summary of pesticide use report data 2012: Indexed by commodity.
Dara, S. K. 2014. New strawberry IPM studies with chemical, botanical, and microbial solutions. CAPCA Adviser 17: 35-37.
National Agricultural Statistics Service (NASS) 2013. California agricultural statistics: 2012 crop year.