- Author: Surendra K. Dara
Mechanisms of insecticide resistance in insects.
Use of biopesticides or non-chemical pesticides is encouraged as a part of integrated pest management (IPM) for environmental and human safety and to reduce the risk of insecticide resistance. With the increase in biopesticide use in both organic and conventional cropping systems, it is a good time to review the potential of insect resistance to botanical and microbial pesticides.
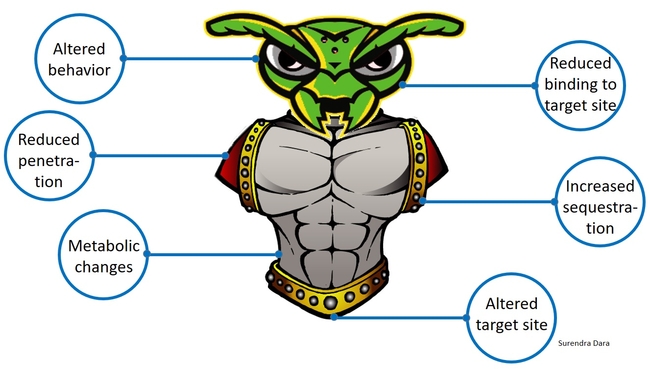
Insects and mites develop resistance to chemical pesticides through genetic, metabolic, or behavioral changes resulting in reduced penetration of toxin, increased sequestration or excretion, reduced binding to the target site, altered target site that prevents binding of the toxin, or reduced exposure to the toxin through modified behavior. When the active ingredient is a toxic molecule and has the mode of action similar to that of a chemical compound, regardless of the plant or microbial origin, arthropods are more likely to develop resistance through one or more of the abovementioned mechanisms. When the mode of action is infection by a microorganism, rather than a toxin, arthropods are less likely to develop resistance. Under natural circumstances, plants, insects, natural enemies, and beneficial or harmful microbes continuously co-evolve and adapt to changing environment. When there is a higher selection pressure, such as indiscriminate use of chemical pesticides, increased mutagenesis can lead to resistance issues. A good understanding of insect resistance to biopesticides will help minimize potential risks and improve their efficient use in IPM.
Resistance to botanical pesticides
Nicotine, an alkaloid from Nicotiana spp., is one of the earlier botanical pesticides known. Although nicotine is not currently used as an insecticide, its synthetic alternatives – neonecotinoids – are commonly used against several pests. Botanical insecticide pyrethrum, extracted from the flowers of Chrysanthemum cinerariaefolium, contains insecticidal pyrethrins (synthetic pyrethrins are referred to as pyrethroids). Although insect resistance to pyrethrum or pyrethroid compounds has been known (Whitehead, 1959; Immaraju et al., 1992; Glenn et al., 1994), they have been effectively used against a number of pests through careful placement in IPM, organic, or conventional management strategies. Additionally, pyrethrin products have been effectively used along with piperonyl butoxide, which acts as a synergist and resistance breaker (Gunning et al. 2015).
Another botanical insecticidal compound, azadirachtin, is a tetranortriterpenoid limonoid from neem (Azadirachta indica) seeds, which acts as an insecticide, antifeedant, repellent and insect growth regulator. While neem oil, which has a lower concentration of azadirachtin, has been used in the United States as a fungicide, acaricide, and insecticide for a long time, several azadirachtin formulations in powder and liquid forms have become popular in recent years and were found effective in managing important pests (Dara 2015a and 2016). Feng and Isman (1995) reported that the green peach aphid, Myzus persicae developed resistance to pure azadirachtin under artificially induced selection pressure after 40 generations, but did not develop resistance to a refined neem seed extract. They suggested that natural blend of azadirachtin compounds in a biopesticide would not exert selection pressure that could lead to resistance. Additionally, Mordue and Nisbet (2000) discussed that azadirachtin can play a role in insecticide resistance management because it reduces the detoxification enzyme production as a protein synthesis inhibitor. Azadirachtin also improved the efficacy of other biopesticides in multiple studies (Trisyono and Whalon, 2000; Dara, 2013 and 2015b).
Insects feeding on plant allelochemicals can develop cross-resistance to insecticides (Després et al., 2007). For example, overproduction of detoxification enzymes such as glutathione S-transferases and monooxygenases in the fall armyworm, Spodoptera frugiperda,when it fed on corn and cowpea, respectively, imparted cross-resistance to various chemical pesticides. It is important to keep this in mind when botanical pesticides are used to detect potential resistance issues.
Resistance to bacterial biopesticides
Bacillus thuringiensis (Bt)is a gram-positive soil bacterium, which contains crystalline toxic protein that is activated upon ingestion by an insect host, binds to the receptor sites in the midgut, and eventually causes insect death. Since the mode of action involves a toxin rather than bacterial infection, several insects developed resistance to Bt pesticides or transgenic crops that contain Bt toxins (Tabashnik et al., 1990; McGaughey and Whalon, 1992; Tabashnik, 1994; Iqbal et al., 1996). However, Bt pesticides are still very popular and used against a variety of lepidopteran (Bt subsp. aizawai and Bt subsp. kurstaki), dipteran (Bt subsp. israelensis and Bt subsp. sphaericus), and coleopteran (Bt subsp. tenebrionis) pests.
Spinosad is a mixture of macrocyclic lactones, spinosyns A and spinosyns D, derived from Saccharopolyspora spinosa, an actinomycete gram-positive bacterium, and is used against dipteran, hymenopteran, lepidopteran, thysanopteran, and other pests. Spinosad products, while naturally derived are registered as chemical pesticides, not as biopesticides. Insect resistance to spinosad later led to the development of spinetoram, which is a mixture of chemically modified spinosyns J and L. Both spinosad and spinetoram are contact and stomach poisons and act on insect nervous system by continuous activation of nicotinic acetylcholine receptors. However, insect resistance to both spinosad (Sayyed et al., 2004; Bielza et al., 2007) and spinetoram (Ahmad and Gull, 2017) has been reported due to extensive use of these pesticides. Cross-resistance between spinosad and some chemical insecticides has also occurred in some insects (Mota-Sanchez et al., 2006; Afzal and Shad, 2017).
Resistance to viral biopesticides
Baculovirus infections in lepidoptera have been known for centuries, especially in silkworms. Currently, there are several commercial formulations of nucleopolyhedroviruses (NPV) and granuloviruses (GV). When virus particles are ingested by the insect host, usually lepidoptera, they invade the nucleii of midgut, fatbody, or other tissue cells and kill the host. Baculoviruses are generally very specific to their host insect species and can be very effective in bringing down the pest populations. However, variations in the susceptibility of certain insect populations and development of resistant to viruses has occurred in several host species (Siegwart et al., 2015). Resistance to different isolates of Cydia pomonella granulovirus (CpGV-M, CpGV-S) in codling moth (Cydia pomonella) populations is well known in Germany and other parts of Europe (Sauer et al., 2017a & b).
Resistance to fungal biopesticides
There are several fungi that infect insects and mites. Fungal infection starts when fungal spores come in contact with an arthropod host. First, they germinate and gain entry into the body by breaching through the cuticle. Fungus later multiplies, invades the host tissues, kills the host, and emerges from the cadaver to produce more spores. Entomophthoralean fungi such as Entomophthora spp., Pandora spp., and Neozygites spp. can be very effective in pest management through natural epizootics, but cannot be cultured in vitro for commercial scale production. Hypocrealean fungi such as Beauveria bassiana, Isarea fumosorosea, Metarhizium brunneum, and Verticillium lecanii,on the other hand, can be mass-produced in vitro and are commercially available. These fungi are comparable to broad-spectrum insecticides and are pathogenic to a variety of soil, foliar, and fruit pests of several major orders. Since botanical, bacterial, and viral biopesticides have insecticidal metabolites, proteins, or viral particles that have specific target sites and mode of action, insects have a higher chance of developing resistance through one or more mechanisms. Although fungi also have insecticidal proteins such as beauvericin in B. bassiana and I. fumosorosea and dextruxin in M. anisopliae and M. brunneum, their mode of action is more through fungal infection and multiplication and arthropods are less prone to developing resistance to entomopathogenic fungi. However, insects can develop resistance to entomopathogenic fungi through increased melanism, phenoloxidase activity, protease inhibitor production, and antimicrobial and antifungal peptide production (Wilson et al., 2001; Zhao et al., 2012; Dubovskiy et al., 2013). It appears that production of detoxification enzymes in insects against fungal infections can also impart resistance to chemical pesticides. Infection of M. anisopliae in the larvae of greater wax moth, Galleria mellonella, increased dexotification enzyme activity and thus resistance to malathion (Serebrov et al., 2006).
These examples show that insects can develop resistance to biopesticides in a manner somewhat similar to chemical pesticides, but due to the typically more complex and multiple modes of action, at a significantly lesser rate depending on the kind of botanical compound or microorganism involved. Resistance to entomopathogenic fungi is less common than with other entomopathogens. Since biopesticide use is not as widespread as chemical pesticides, the risk of resistance development is less for the former. However, excessive use of any single tool has the potential for resistance or other issues and IPM, which uses a variety of management options, is always a good strategy.
Acknowledgements: Thanks to Pam Marrone for reviewing the manuscript.
References
Afzal, M.B.S. and S. A. Shad. 2017. Spinosad resistance in an invasive cotton mealybug, Phenacoccus solenopsis: cross-resistance, stability and relative fitness. J. Asia-Pacific Entomol. 20: 457-462.
Ahmad M. and S. Gull. 2017. Susceptibility of armyworm Spodoptera litura (Lepidoptera: Noctuidae) to novel insecticides in Pakistan. The Can. Entomol. 149: 649-661.
Bielza, P., V. Quinto, E. Fernández, C. Grávalos, and J. Contreras. 2007. Genetics of spinosad resistance in Frankliniella occidentalis (Thysanoptera: Thripidae). J. Econ. Entomol. 100: 916-920.
Dara, S. K. 2013. Strawberry IPM study 2012: managing insect pests with chemical, botanical, and microbial pesticides. UCANR eJournal Strawberries and Vegetables March 13, 2013 (http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=9595).
Dara, S. K. 2015a. Root aphids and their management in organic celery. CAPCA Adviser 18(5): 65-70.
Dara, S. K. 2015b. Strawberry IPM study 2015: managing insect pests with chemical, botanical, microbial, and mechanical control options. UCANR eJournal Strawberries and Vegetables November 30, 2015 (http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=19641).
Dara, S. K. 2016. Managing strawberry pests with chemical pesticides and non-chemical alternatives. Intl. J. Fruit Sci. https://doi.org/10.1080/15538362.2016.1195311
Després, L., J.-L. David, and C. Gallet. 2007. The evolutionary ecology of insect resistance to plant chemicals. Trends in Ecol. Evol. 22: 298-307.
Dubovskiy, I. M., M.M.A. Whitten, O. N. Yaroslavtseva, C. Greig, V. Y. Kryukov, E. V. Grizanova, K. Mukherjee, A. Vilcinskas, V. V. Glupov, and T. M. Butt. 2013. Can insects develop resistance to insect pathogenic fungi? PloS one 8: e60248.
Feng, R. and M. B. Isman. 1995. Selection for resistance to azadirachtin in the green peach aphid, Myzus persicae. Experientia 51: 831-833.
Glenn, D. C., A. A. Hoffmann, and G. McDonald. 1994. Resistance to pyrethroids in Helicoverpa armigera (Lepidoptera: Noctuidae) from corn: adult resistance, larval resistance, and fitness effects. J. Econ. Entomol. 87: 1165-1171.
Gunning, R., G. Moores, and M. Balfe. 2015. Novel use of pyrethrum to control resistant insect pests on cotton. Acta Hort. 1073: 113-118. https://doi.org/10.17660/ActaHortic.2015.1073.16
Immaraju, J. A., T. D. Paine, J. A. Bethke, K. L. Robb, and J. P Newman. 1992. Western flower thrips (Thysanoptera: Thripidae) resistance to insecticides in coastal California greenhouses. J. Econ. Entomol. 85: 9-14.
Iqbal, K., R.H.J. Vekerk, M. J. Furlong, P. C. Ong, S. A. Rahman, and D. J. Wright. 1996. Evidence for resistance to Bacillus thuringiensis (Bt) subsp. kurstaki HD-1, Bt subsp. aizawai and Abamectin in field populations of Plutella xylostella from Malaysia. Pest Manag. Sci. 48: 89-97.
McGaughey, W. H. and M. E. Whalon. 1992. Managing insect resistance to Bacillus thuringiensis toxins. Science 258: 1451-1455.
Mordue, A. J. and A. J. Nisbet. 2000. Azadirachtin from the neem tree Azadirachta indica: its action against insects. An. Soc. Entomol. Bras. 29: 615-632.
Mota-Sanchez, D., R. M. Hollingworth, E. J. Grafius, and D. D. Moyer. 2006. Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Pest Manag. Sci. 62: 30-37.
Sauer, A. J., E. Fritsch, K. Undorf-Spahn, P. Nguyen, F. Marec, D. G. Heckel, and J. A. Jehle. 2017a. Novel resistance to Cydia pomonella granulovirus (CpGV) in codling moth shows autosomal and dominant inheritance and confers cross-resistance to different CpGV genome groups. PLoS ONE 12(6): e0179157.
Sauer, A. J., S. Schulze-Bopp, E. Fritsch, K. Undorf-Spahn, and J. A. Jehle. 2017b. A third type of resistance of codling moth against Cydia pomonella granulovirus (CpGV) shows a mixture of a Z-linked and autosomal inheritance pattern. Appl. Environ. Microbiol. AEM-01036.
Sayyed, A. H., D. Omar, and D. J. Wright. 2004. Genetics of spinosad resistance in a multi-resistant field-selected population of Plutella xylostella. Pest Manag. Sci. 60: 827-832.
Serebrov, V. V., O. N. Gerber, A. A. Malyarchuk, V. V. Martemyanov, A. A. Alekseev, and V. V. Glupov. Effect of entomopathogenic fungi on detoxification enzyme activity in greater wax moth Galleria mellonella L. (Lepidoptera, Pyralidae) and role of detoxification enzymes in development of insect resistance to entomopathogenic fungi. Anim. Human Physiol. 33: 581-586.
Siegwart, M., B. Graillot, C. B. Lopez, S. Besse, M. Bardin, P. C. Nicot, and M. Lopez-Ferber. 2015. Resistance to bio-insecticides or how to enhance their sustainability: a review. Front. Plant Sci. 6:381. https://doi.org/10.3389/fpls.2015.00381
Tabashnik, B. 1994. Evolution of resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 39: 47-79.
Tabashnik, B. E., N. L. Cushing, N. Finson, and M. W. Johnson. 1990. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 83: 1671-1676.
Trisyono, A. and M. E. Whalon. 2000. Toxicity of neem applied alone and in combination with Bacillus thuringiensis to Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 92: 1281-1288.
Whitehead, G. B. 1959. Pyrethrum resistance conferred by resistance to DDT in the blue tick. Nature 184: 378-379.
Wilson K., S. C. Cotter, A. F. Reeson, and J. K. Pell. 2003. Melanism and disease resistance in insects. Ecology Letters 4: 637-649.
Zhao, P., Z. Dong, J. Duan, G. Wang, L. Wang, Y. Li, Z. Xiang, and Q. Xia. 2012. Genome-wide identification and immune response analysis of serine protease inhibitor genes in the silkworm, Bombyx mori. PloS one 7: e31168.
- Author: Surendra K. Dara
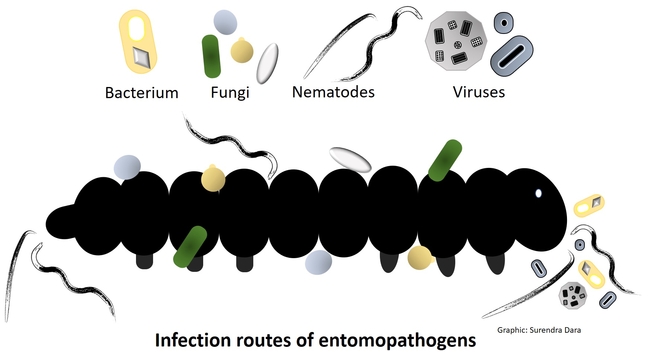
Entomopathogens are microorganisms that are pathogenic to arthropods such as insects, mites, and ticks. Several species of naturally occurring bacteria, fungi, nematodes, and viruses infect a variety of arthropod pests and play an important role in their management. Some entomopathogens are mass-produced in vitro (bacteria, fungi, and nematodes) or in vivo (nematodes and viruses) and sold commercially. In some cases, they are also produced on small scale for non-commercial local use. Using entomopathogens as biopesticides in pest management is called microbial control, which can be a critical part of integrated pest management (IPM) against several pests.
Some entomopathogens have been or are being used in a classical microbial control approach where exotic microorganisms are imported and released for managing invasive pests for long-term control. The release of exotic microorganisms is highly regulated and is done by government agencies only after extensive and rigorous tests. In contrast, commercially available entomopathogens are released through inundative application methods as biopesticides and are commonly used by farmers, government agencies, and homeowners. Understanding the mode of action, ecological adaptations, host range, and dynamics of pathogen-arthropod-plant interactions is essential for successfully utilizing entomopathogen-based biopesticides for pest management in agriculture, horticulture, orchard, landscape, turf grass, and urban environments.
Entomopathogen groups
Important entomopathogen groups and the modes of their infection process are described below.
Bacteria
There are spore-forming bacterial entomopathogens such as Bacillus spp., Paenibacillus spp., and Clostridium spp, and non-spore-forming ones that belong to the genera Pseudomonas, Serratia, Yersinia, Photorhabdus, and Xenorhabdus. Infection occurs when bacteria are ingested by susceptible insect hosts. Pseudomonas, Serratia and Yersinia are not registered in the USA for insect control.Several species of the soilborne bacteria, Bacillus and Paenibacillus are pathogenic to coleopteran, dipteran, and lepidopteran insects. Bacillus thuringiensis subsp. aizawai, Bt subsp. kurstaki, Bt subsp. israelensis, Bt subsp. sphaericus, and Bt subsp. tenebrionis are effectively used for controlling different groups of target insects. For example, Bt subsp. aizawai and Bt subsp. kurstaki are effective against caterpillars, Bt subsp. israelensis and Bt subsp. sphaericus target mosquito larvae, and Bt subsp. tenebrionis is effective against some coleopterans.
When Bt is ingested, alkaline conditions in the insect gut (pH 8-11) activate the toxic protein (delta-endotoxin) that attaches to the receptors sites in the midgut and creates pore in midgut cells. This leads to the loss of osmoregulation, midgut paralysis, and cell lysis. Contents of the gut leak into insect's body cavity (hemocoel) and the blood (hemolymph) leaks into the gut disrupting the pH balance. Bacteria that enter body cavity cause septicemia and eventual death of the host insect. Insects show different kinds of responses to Bt toxins depending on the crystal proteins (delta-endotoxin), receptor sites, production of other toxins (exotoxins), and requirement of spore. The type responses below are based on the susceptibility of caterpillars to Bt toxins.
Type I response – Midgut paralysis occurs within a few minutes after delta-endotoxin is ingested. Symptoms include cessation of feeding, increase in hemolymph pH, vomiting, diarrhea, and sluggishness. General paralysis and septicemia occur in 24-48 hours resulting in the death of the insect. Examples of insects that show Type I response include silkworm, tomato hornworm, and tobacco hornworm.
Type II response – Midgut paralysis occurs within a few minutes after the ingestion of delta-endotoxin, but there will be no general paralysis. Septicemia occurs within 24-72 hours. Examples include inchworms, alfalfa caterpillar, and cabbage butterfly.
Type III response – Midgut paralysis occurs after delta-endotoxin is ingested followed by cessation of feeding. Insect may move actively as there will be no general paralysis. Mortality occurs in 48-96 hours. Higher mortality occurs if spores are ingested. Insect examples include Mediterranean flour moth, corn earworm, gypsy moth, spruce budworm.
Type IV response – Insects are naturally resistant to infection and older instars are less susceptible than the younger ones. Midgut paralysis occurs after delta-endotoxin is ingested followed by cessation of feeding. Insect may move actively as there will be no general paralysis. Mortality occurs in 72-96 or more hours. Higher mortality occurs if spores are ingested. Cutworms and armyworms are examples for this category.
Unlike caterpillars, the response in mosquitoes is different where upon ingestion of Bt subsp. israelensis delta-endotoxin, the mosquito larva is killed within 20-30 min.
While Bt with its toxic proteins is very effective as a biopesticide against several pests, excessive use can lead to resistance development. Corn earworm, diamondback moth, and tobacco budworm are some of the insects that developed resistance to Bt toxins. Genetic engineering allowed genes that express Bt toxins to be inserted into plants such as corn, cotton, eggplant, potato, and soybean and reduced the need to spray pesticides. However, appropriate management strategies are necessary to reduce insect resistant to Bt toxins in transgenic plants.
Paenibacillus popilliae is commonly used against Japanese beetle larvae and known to cause the milky spore disease. Although Serratia is not registered for use in the USA, a species is registered for use against a pasture insect in New Zealand. In the case of Photorhabdus spp. and Xenorhabdus spp., which live in entomopathogenic nematodes symbiotically, bacteria gain entry into the insect host through nematodes. Biopesticides based on heat-killed Chromobacterium subtsugae and Burkholderia rinojensis are reported to have multiple modes of action and target mite and insect pests of different orders.
Fungi
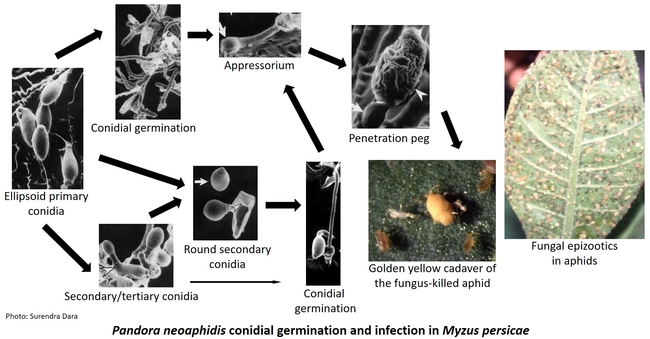
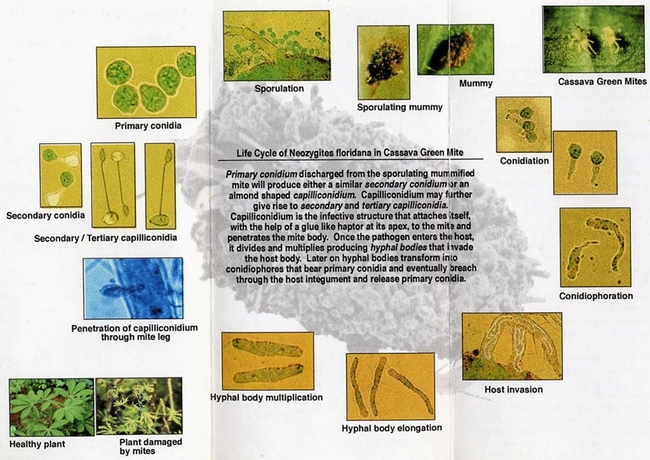
Entomopathogenic fungi typically cause infection when spores come in contact with the arthropod host. Under ideal conditions of moderate temperatures and high relative humidity, fungal spores germinate and breach the insect cuticle through enzymatic degradation and mechanical pressure to gain entry into the insect body. Once inside the body, the fungi multiply, invade the insect tissues, emerge from the dead insect, and produce more spores. Natural epizootics of entomophthoralean fungi such as Entomophaga maimaiga (in gypsy moth), Entomophthora muscae (in flies), Neozygites fresenii (in aphids), N. floridana (in mites), and Pandora neoaphidis (in aphids) are known to cause significant reductions in host populations. Although these fastidious fungi are difficult to culture in artificial media and do not have the potential to be sold as biopesticides they are still important in natural control of some pest species. Hypoclealean fungi such as Beauveria bassiana, Isaria fumosorosea, Hirsutella thompsonii, Lecanicillium lecanii, Metarhizium acridum, M. anisopliae, and M. brunneum, on the other hand, are commercially sold as biopesticides in multiple formulations around the world. Fungal pathogens have a broad host range and are especially suitable for controlling pests that have piercing and sucking mouthparts because spores do not have to be ingested. However, entomopathogenic fungi are also effective against a variety of pests such as wireworms and borers that have chewing mouthparts.
Related to fungi, the spore-forming microsporidium, Paranosema (Nosema) locustae is a pathogen that has been used for controlling locusts, grasshoppers, and some crickets. When P. locustae is ingested, the midgut tissues become infected, followed by infection in the fat body tissues. The disease weakens and eventually kills the orthopteran host within a few weeks.
Various insects killed by different species of entomopathogenic fungi
Nematodes
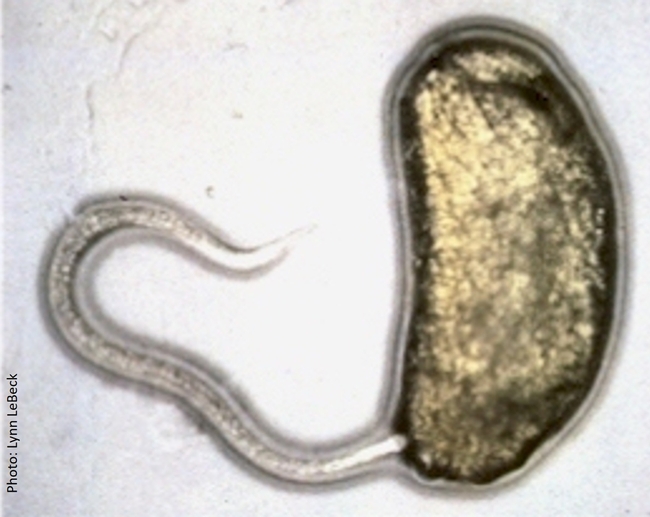
Entomopathogenic nematodes are microscopic, soil-dwelling worms that are parasitic to insects. Several species of Heterorhabditis and Steinernema are available in multiple commercial formulations, primarily for managing soil insect pests. Infective juveniles of entomopathogenic nematodes actively seek out their hosts and enter through natural openings such as the mouth, spiracles, and anus or the intersegmental membrane. Once inside the host body, the nematodes release symbiotic bacteria that kill the host through bacterial septicemia. Heterorhabditis spp. carry Photorhabdus spp. bacteria and Steinernema spp. carry Xenorhabdus spp. bacteria. Phasmarhabditis hermaphrodita is also available for controlling slugs in Europe, but not in the USA.
Infective juvenile of Steinernema carpocapsae entering the first instar larva of a leafminer through its anus.
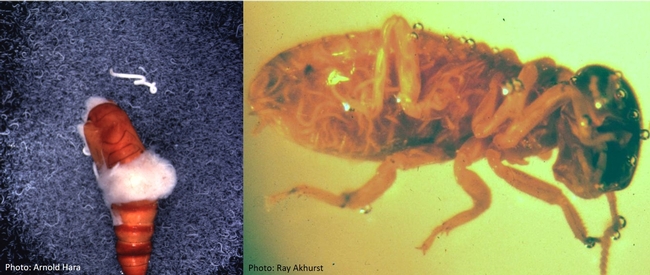
Nematodes in beet armyworm pupa (left) and termite worker (right).
Viruses
Similar to bacteria, entomopathogenic viruses need to be ingested by the insect host and therefore are ideal for controlling pests that have chewing mouthparts. Several lepidopteran pests are important hosts of baculoviruses including nucleopolyhedroviruses (NPV) and granuloviruses (GV). These related viruses have different types of occlusion bodies in which the virus particles (virions) are embedded. Virus particles invade the nucleus of the midgut, fat body or other tissue cells, compromising the integrity of the tissues and liquefying the cadavers. Before death, infected larvae climb higher in the plant canopy, which aids in the dissemination of virus particles from the cadavers to the lower parts of the canopy. This behavior aids in the spread of the virus to cause infection in healthy larvae. Viruses are very host specific and can cause significant reduction of host populations. Examples of some commercially available viruses include Helicoverpa zea single-enveloped nucleopolyhedrovirus (HzSNVP), Spodoptera exigua multi-enveloped nucleopolyhedrovirus (SeMNPV), and Cydia pomonella granulovirus (CpGV).
Most entomopathogens typically take 2-3 days to infect or kill their host except for viruses and P. locustae which take longer. Compared to viruses (highly host specific) and bacteria (moderately host specific), fungi generally have a broader host range and can infect both underground and aboveground pests. Because of the soil-dwelling nature, nematodes are more suitable for managing soil pests or those that have soil inhabiting life stages.
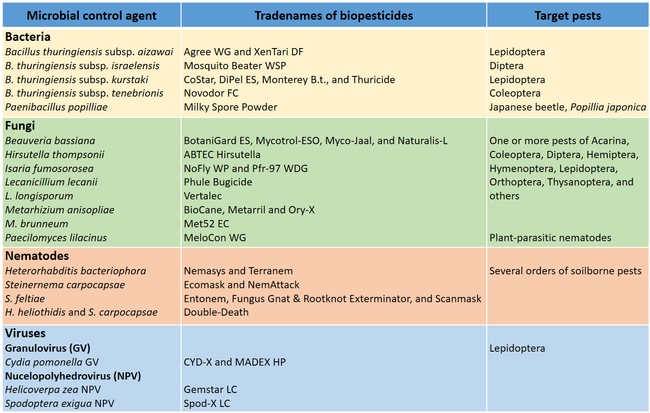
Biopesticides based on various entomopathogenic microorganisms and their target pests
Microbial control and Integrated Pest Management
There are several examples of entomopathogen-based biopesticides that have played a critical role in pest management. Significant reduction in tomato leaf miner, Tuta absoluta, numbers and associated yield loss was achieved by Bt formulations in Spain (Gonzalez-Cabrera et al, 2011). Bt formulations are also recommended for managing a variety of lepidopteran pests on blueberry, grape, and strawberry (Haviland, 2014; Zalom et al, 2014; Bolda and Bettiga, 2014; Varela et al, 2015).
Lecanicellium muscarium-based formulation reducedgreenhouse whitefly (Trialeurodes vaporariorum) populations by 76-96% in Mediterranean greenhouse tomato (Fargues et al, 2005). In other studies, B. bassiana applications resulted in a 93% control of twospotted spider mite (Tetranychus urticae) populations in greenhouse tomato (Chandler et al, 2005) and 60-86% control on different vegetables (Gatarayiha et al, 2010). The combination of B. bassiana and azadirachtin reduced rice root aphid (Rhopalosiphum rufiabdominale) and honeysuckle aphid (Hyadaphis foeniculi) populations by 62% in organic celery in California (Dara, 2015a). Chromobacterium subtsugae and B. rinojensis caused a 29 and 24% reduction, respectively, in the same study. IPM studies in California strawberries also demonstrated the potential of entomopathogenic fungi for managing the western tarnished plant bug (Lygus hesperus) and other insect pests (Dara, 2015b, 2016). Entomopathogenic fungi also have a positive effect on promoting drought tolerance or plant growth as seen in cabbage (Dara et al, 2016) and strawberry (Dara, 2013) and antagonizing plant pathogens (Dara et al, 2017)
Application of SeMNPV was as efficacious as methomyl and permithrin in reducing beet armyworms (S. exigua) in head lettuce in California (Gelernter et al, 1986). Several studies demonstrated PhopGV as an important tool for managing the potato tubermoth (Phthorimaea operculella) (Lacey and Kroschel, 2009).
The entomopathogenic nematode, S. feltiae,reduced raspberry crown borer (Pennisetia marginata) populations by 33-67% (Capinera et al, 1986). For managing the branch and twig borer (Melagus confertus) in California grapes, S. carpocapsae is one of the recommended options (Valera et al, 2015).
Entomopathogens can be important tools in IPM strategies in both organic and conventional production systems. Depending on the crop, pest, and environmental conditions, entomopathogens can be used alone or in combination with chemical, botanical pesticides or other entomopathogens.
Acknowledgements: Thanks to Dr. Harry Kaya for reviewing this article.
References
Bolda, M. P. and L. J. Bettiga. 2015. UC IPM Pest Management Guidelines: Caneberries. UC ANR Pub. 3437.
Capinera, J. L., W. S. Cranshaw, and H. G. Hughes. 1986. Suppression of raspberry crown borer Pennisetia marginata (Harris) (Lepidoptera: Sesiidae) with soil applications of Steinernema feltiae (Rhabditida:Steinernematidae). J. Invertebr. Pathol. 48: 257-258.
Chanlder, D., G. Davidson, and R. J. Jacobson. 2005. Laboratory and glasshouse evaluation of entomopathogenic fungi angainst the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae), on tomato, Lycopersicon esculentum. Biocon. Sci. Tech. 15: 37-54.
Dara, S. K. 2013. Entomopathogenic fungus Beauveria bassiana promotes strawberry plant growth and health. UCANR eJournal Strawberries and Vegetables, 30 September, 2013. (http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=11624)
Dara, S. K. 2015a. Reporting the occurrence of rice root aphid and honeysuckle aphid and their management in organic celery. UCANR eJournal Strawberries and Vegetables, 21 August, 2015. (http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=18740)
Dara, S. K. 2015b. Integrating chemical and non-chemical solutions for managing lygus bug in California strawberries. CAPCA Adviser 18 (1) 40-44.
Dara, S. K. 2016. IPM solutions for insect pests in California strawberries: efficacy of botanical, chemical, mechanical, and microbial options. CAPCA Adviser 19 (2): 40-46.
Dara, S. K., S.S.R. Dara, and S.S. Dara. 2016. First report of entomopathogenic fungi, Beauveria bassiana, Isaria fumosorosea, and Metarhizium brunneum promoting the growth and health of cabbage plants growing under water stress. UCANR eJournal Strawberries and Vegetables, 19 September, 2016. (http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=22131)
Dara, S.S.R., S. S. Dara, S. K. Dara, and T. Anderson. 2017. Fighting plant pathogenic fungi with entomopathogenic fungi and other biologicals. CAPCA Adviser 20 (1): 40-44.
Fargues, J., N. Smits, M. Rougier, T. Boulard, G. Rdray, J. Lagier, B. Jeannequin, H. Fatnassi, and M. Mermier. 2005. Effect of microclimate heterogeneity and ventilation system on entomopathogenic hyphomycete infectiton of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) in Mediterranean greenhouse tomato. Biological Control 32: 461-472.
Gatarayiha, M. C., M. D. Laing, and M. Ray. 2010. Effects of adjuvant and conidial concentration on the efficacy of Beauveria bassiana for the control of the two-spotted spider mite, Tetranychus urticae. Exp. Appl. Acarol. 50: 217-229.
Gelernter, W. D., N. C. Toscano, K. Kido, and B. A. Federici. 1986. Comparison of a nuclear polyhedrosis virus and chemical insecticides for control of the beet armyworm (Lepidopter: Noctuidae) on head lettuce. J. Econ. Entomol. 79: 714-717.
González-Cabrera, J., J. Mollá, H. Monton, A. Urbaneja. 2011. Efficacy of Bacillus thuringiensis (Berliner) in controlling the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). BioControl 56: 71–80.
Haviland, D. R. 2014. UC IPM Pest Management Guidelines: Blueberry. UC ANR Pub. 3542.
Lacey, L. A. and J. Kroschel. 2009. Microbial control of the potato tuber moth (Lepidoptera: Gelechiidae). Fruit Veg. Cereal Sci. Biotechnol. 3: 46-54.
Varela, L. G., D. R. Haviland, W. J., Bentley, F. G. Zalom, L. J. Bettiga, R. J. Smith, and K. M. Daane. 2015. UC IPM Pest Management Guidelines: Grape. UC ANR Pub. 3448.
Zalom, F. G., M. P. Bolda, S. K. Dara, and S. Joseph. 2014. UC IPM Pest Management Guidelines: Strawberry. UC ANR Pub. 3468.