Calag Archive
Calag Archive
Grape erineum mite: Postharvest sulfur use reduces subsequent leaf blistering
Publication Information
California Agriculture 74(2):94-100. https://doi.org/10.3733/ca.2020a0012
Published online June 09, 2020
PDF | Citation | Permissions
NALT Keywords
Abstract
The occurrence of eriophyid mites (Calepitrimerus vitis [rust mites] and Colomerus vitis [erineum mites and bud mites]) in vineyards worldwide is associated with leaf deformation, stunted shoot growth and reduced yield potential. In the North Coast region of California, leaf blistering by the erineum strain of Colomerus vitis is the most widespread symptom of eriophyid mite damage. Unlike rust and bud mites, erineum mites are generally considered a nuisance pest that is incidentally controlled by sulfur-dominated management programs for powdery mildew. However, recent reductions in the use of sulfur have allowed erineum mite populations to expand, highlighting the need for alternative management options. In this study, we posited that, during autumn, mites moving to buds from erinea (leaf blisters) to overwinter could be susceptible to sulfur applications. During four growing seasons, we documented patterns of mite movement to identify key sulfur application timing. We found the greatest numbers of migrating erineum mites from late September to early November. Concurrently, in replicated trials, we evaluated the efficacy of postharvest sulfur applications to reduce blistering. Sulfur applied during the migration period in 2013 appeared to eradicate leaf blistering in the 2014 growing season. In subsequent trials, sulfur treatments reduced blistering to less than 10% incidence, compared to 40% to 50% incidence in control plots.
Full text
The grapevine eriophyid mite group (Acari: Eriophyidae) includes the rust mite (Calepitrimerus vitis), the bud mite and the blister (or erineum) mite. The bud and blister mites, both Colomerus vitis, are morphologically similar but genetically distinct (Carew et al. 2004). Rust mite feeding, which results in malformed leaves, incomplete cluster formation and severely stunted, scarred and deformed shoots, can cause economic losses that reach as high as 23% (Walton et al. 2007). Damage from the bud mite can be equally severe (Bernard et al. 2005; Carew et al. 2004), requiring management interventions such as modified pruning strategies (Dennill 1991) or pesticide applications (Bernard et al. 2005). In contrast, damage resulting from the blister or erineum mite is limited to the formation of blisters (erinea) on grapevine leaves (Carew et al. 2004). Although these blisters may affect the photosynthetic capacity of the vines (Carew et al. 2004), the erineum strain has generally been considered a nuisance pest, except to certain grapevine cultivars (Khederi et al. 2018), or when elevated populations cause stress to young vines in the field (Varela et al. 2013) or in plant propagation facilities (Ferragut et al. 2008). Recently, however, erineum mites have been implicated as a potential vector of grapevine pinot gris virus (GPGV) (Malagnini et al. 2016), a pathogen whose damage is characterized by leaf mottling, deformation and stunted shoot growth (Saldarelli et al. 2015). As with other arthropod-transmitted diseases of grapevine (Almeida et al. 2013; Daugherty et al. 2015), disease management efforts for GPGV may incorporate a vector management component to reduce populations of erineum mite.
Leaf blistering by grape erineum mite. UC Cooperative Extension trials conducted in Napa County vineyards demonstrated that applying sulfur in late September through early November significantly reduced the incidence of leaf blistering in the subsequent growing season.
Erineum mite is widely distributed in vineyards throughout California, in both coastal and interior locations (Smith and Stafford 1948). (In the Pacific Northwest and Australia, on the other hand, rust and bud mites predominate [Walton et al. 2007; Duso et al. 2010].) Erineum mite damage is characterized by the formation on the upper leaf surface of elevated leaf galls or blisters (erinea), from which the mite gets its common name. Plant hairs grow profusely on the lower leaf surface in the galled area; hairs are white in young galls, aging to a reddish-brown color in older galls, as the leaf hairs die. These distinctive galls are readily identifiable, and form only on newly emerging leaves. Once leaves reach a diameter greater than 0.5 inches (1.3 centimeters), they are no longer susceptible to infection (Smith and Stafford 1948). In the North Coast American Viticultural Area — composed of Napa County and five other counties — damage to young leaves is commonly observed early in the season (prebloom); later in the season (during berry ripening), blisters are often found on leaves at the shoot tip and on lateral shoots. Both bud and erineum mites overwinter as adults in grapevine buds, where they remain active and feed on dormant bud tissue (Carew et al. 2004). After budbreak, the bud mite moves to newly developing buds, whereas erineum mites exit the buds to feed on the leaves. Consequently, in autumn, erineum mites must migrate from the leaves back to the buds to overwinter.
(A) Profuse growth of plant hairs on the underside of a grape leaf resulting from erineum mite feeding (white hairs indicate young galls). (B) In older galls, the leaf hairs darken to a reddish brown.
While phytoseiid mites may provide biological control of erineum mite populations under some conditions (James and Whitney 1993; Ferragut et al. 2008), sulfur applications for grapevine powdery mildew (Erysiphe necator) generally provide incidental control of erineum mite (Smith and Stafford 1948). The authors, however, have recently observed a shift toward reduced sulfur use during the growing season in the North Coast region, driven both by increased reliance on alternate products (such as oils) early in the season and by external factors aimed at limiting the use of sulfur in vineyards. One consequence has been an increased prevalence of leaf blistering, both early and late in the season. As an alternative to in-season treatments, we proposed in this study that sulfur applications in autumn (after harvest, but before leaf fall) could target erineum mites as they moved to the buds to overwinter. We therefore initiated this study (1) to document erineum mite migration patterns in the autumn and to identify periods of elevated activity in the North Coast region and (2) to evaluate the efficacy of an autumn sulfur application in reducing leaf blistering in the subsequent growing season.
Autumn migration patterns of erineum mites
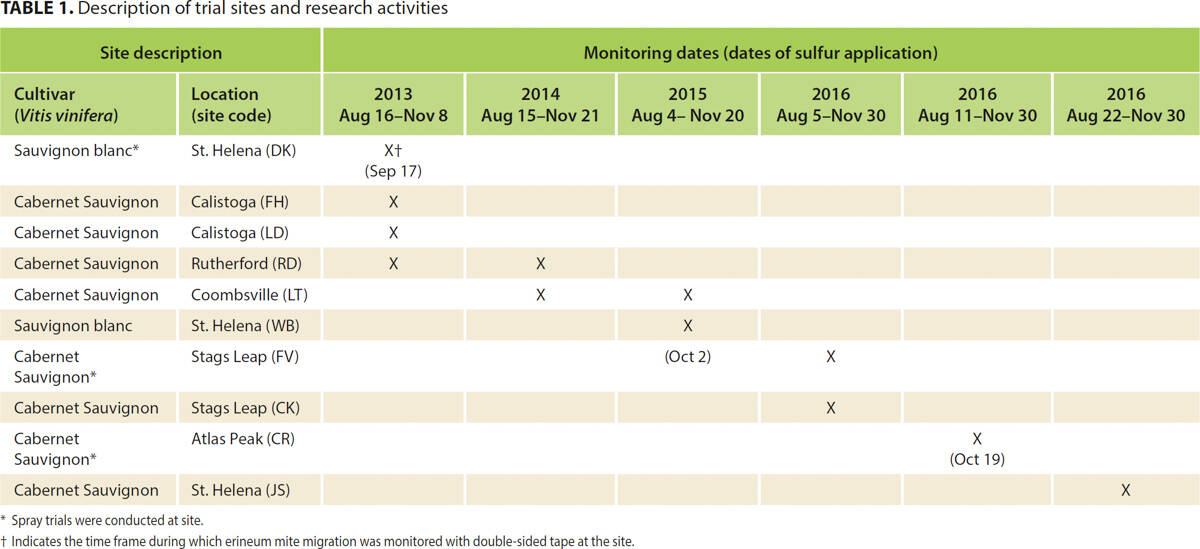
We tracked patterns of erineum mite movement during the late summer and autumn of 2013, 2014, 2015 and 2016. In 2013 and 2016, we monitored four unique vineyard sites and in 2014 and 2015 we monitored two unique vineyard sites (table 1). At all sites, the predominant symptom of eriophyid mite damage was leaf blistering associated with erineum mite. Symptoms of rust or bud mite damage, including malformed leaves and shoots, reduced shoot growth and reduced cluster number, were not present on any of the vines in any of the study years. In 2013, we monitored six vines at each site, and in subsequent years we monitored 12 vines per site. On each monitored vine, we selected three shoots with obvious leaf blistering and deployed double-sided tape (Scotch, St. Paul, Minnesota) at one location on each of the selected shoots, corresponding to (1) basal, (2) middle and (3) upper shoot positions. We defined the basal position as the internode between the clusters, of which there were generally two, on the shoot. Depending on the year, the middle position was two (2015, 2016) or five (2013, 2014) internodes up from the basal position (internode 5 or 8, counting from the base of the shoot). The upper position was five internodes up from the middle position (internode 10 or 13, counting from the base of the shoot). Monitoring was initiated from early August to mid-August; tapes were changed on a 7- to 10-day interval and monitoring concluded around leaf fall (mid-November to late November) or when mites were no longer detected (table 1).
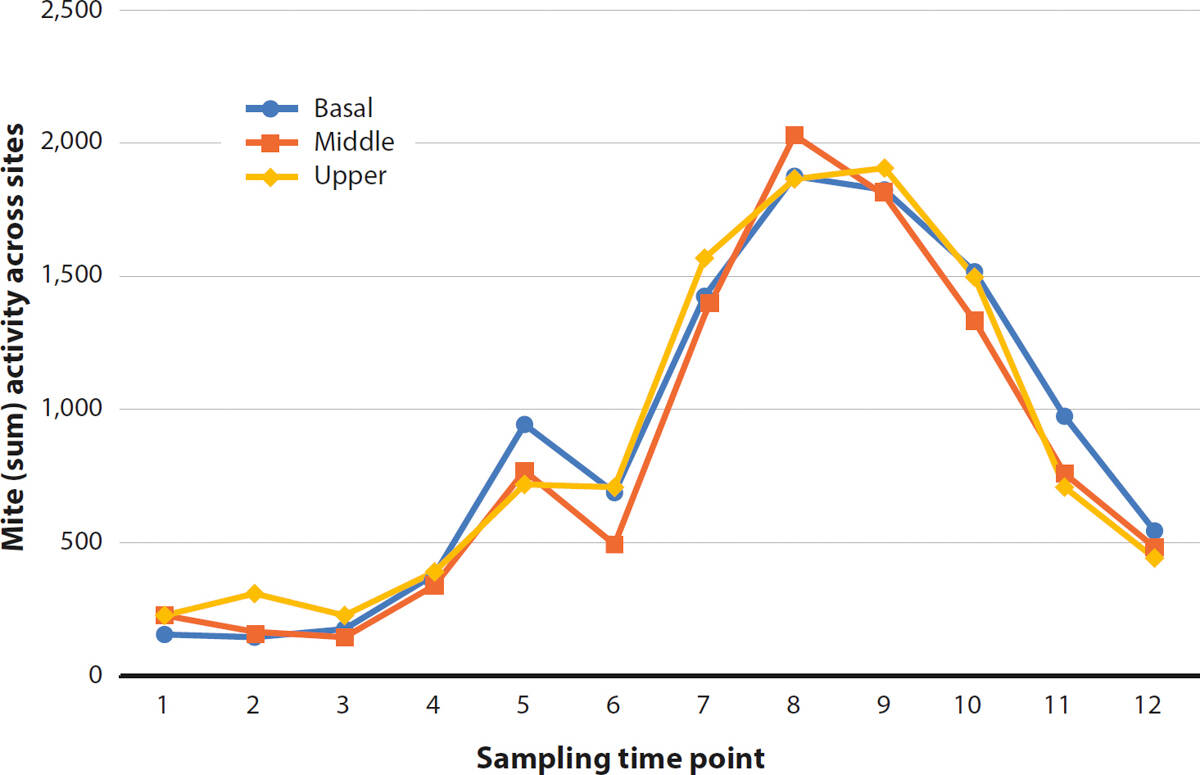
Because it was not feasible to individually count erineum mites, a score representing the absolute number of mites on each tape was generated. Mite activity was quantified by overlaying a grid measuring 2.36 inches (6 centimeters) by 0.79 inches (2 centimeters) on each tape and judging the coverage of mites in each square using a 5-point scale (0 = no mites; 1 = up to 5% coverage; 2 = 6% to 33%; 3 = 34% to 66%; 4 = 67% to 100%). Each tape was then scored by summing the ratings, yielding a single score per tape. Across sites and study years, 12 tapes were missing (that is, they had fallen off the vine prior to collection). In order to avoid distorting overall scores on days when tapes were missing, we calculated average scores and inserted them in place of the missing data. In addition, we calculated total (summed) mite activity at each vineyard site per monitoring date (fig. 1). To assess annual trends in migration patterns, mite activity was compared to calendar date and average heat accumulation, measured in terms of growing degree days (GDD) for grapevines. The GDD model was initiated on April 1 of each growing season. The lower threshold was set at 50°F (10°C) and temperature data was collected from a network of private weather stations operating in Napa County. Significant seasonal differences in mite activity were explored at each site by analyzing mean tape scores using repeated-measures ANOVA and post hoc t-tests to explore changes in activity on specific dates/degree days (see “Data analysis” section at end of article). A secondary analysis then used ANOVA to test for significant differences between shoot positions (basal versus middle versus upper); for these comparisons, data were aggregated across sites for each year to generate sufficient data points in each case.
FIG. 1. Total erineum mite activity on double-sided monitoring tape at each vineyard, with optimal sulfur application (OSA) indicated by the shaded area(s). CK = Stags Leap; CR = Atlas Peak; DK = St. Helena; FH = Calistoga; FV = Stags Leap; JS = St. Helena; LD = Calistoga; LT = Coombsville; RD = Rutherford; WB = St. Helena.
Postharvest sulfur applications
In 2013, 2015 and 2016, we established trials to evaluate the impact of postharvest sulfur applications on the incidence of leaf blistering in the following growing season. We compared one application of dry flowable sulfur at a rate of 5 pounds per acre (in 75 gallons of water) to an untreated control. Because sulfur may also be formulated as a dust for powdery mildew management, the 2016 trial included an additional treatment: 12 pounds per acre of dusting sulfur. Treatments were applied after harvest (table 1) and each treatment was applied over 10 rows and replicated three times at each site. Pretreatment monitoring was conducted in the growing season in which the treatment was applied (with the exception of 2013) and posttreatment monitoring was conducted at two or three time points in the growing season following the treatment. All monitoring was conducted in the two middle rows of each treatment area. On each monitoring date, 30 vines per replicate (90 vines per treatment) were inspected and the presence or absence of blisters was recorded on 10 leaves per vine (five leaves at each of two unique node positions corresponding to basal and upper shoot positions).
For the purposes of analysis, these 10 observations were combined to create a mean score (blister value) for each vine. Mean blister scores were then calculated at each date for each treatment group. For the 2013 trial, a 2 × 3 mixed ANOVA was conducted [Treatment (control versus dry flowable sulfur) x Monitoring Date (posttreatment 1 versus posttreatment 2 versus posttreatment 3)]. Another 2 × 3 ANOVA was run for the 2015 trial; the monitoring date comparisons were pretreatment versus posttreatment 1 versus posttreatment 2. This became a 3 × 3 mixed ANOVA for the 2016 trial due to an additional dusting sulfur treatment.
Erineum mite autumn migration patterns
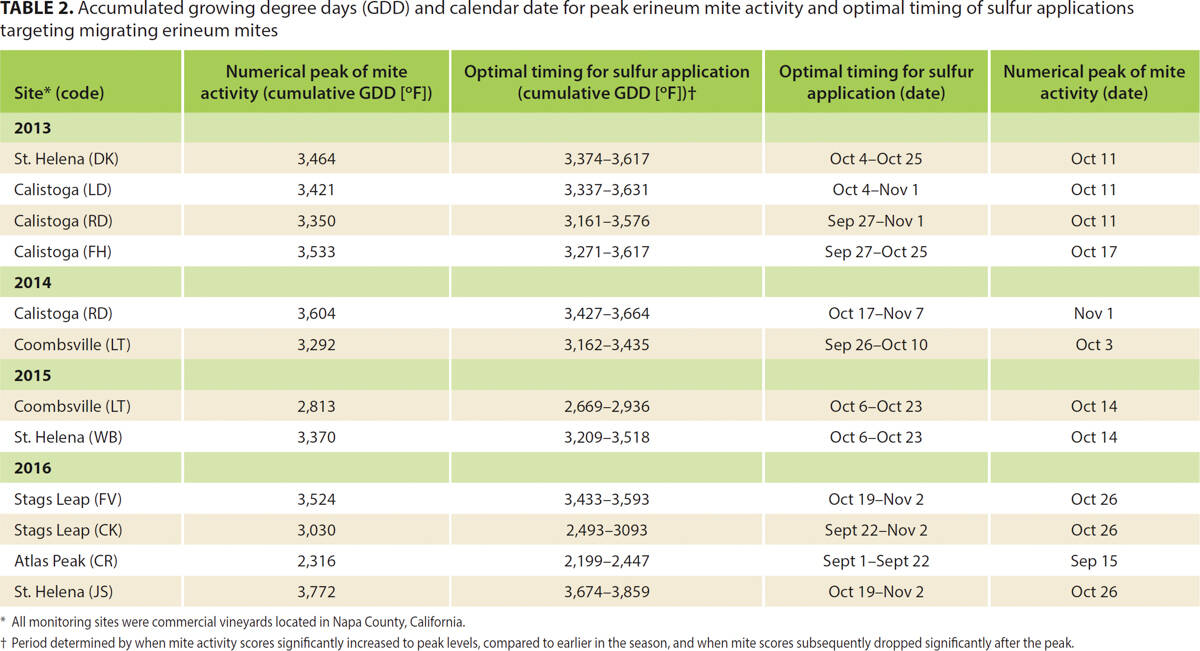
Aside from one site in Rutherford (RD), where mites were not detected until Aug. 29, 2013 mites were found in all vineyard sites at the time that monitoring was initiated (no earlier than Aug. 11 or 1,687 degree days); mites increased to a peak before declining to (near) zero at the end of the monitoring period (no later than Nov. 30 or 4,073 degree days). Across all study periods, mite activity did not differ significantly between shoot positions, with very similar activity observed throughout the canopy (fig. 2). Peak mite activity across sites aligned more closely with calendar date than with accumulated GDD, likely due to the importance of photoperiod as the most predictable environmental indicator of changing seasons and hence the trigger for migration and dormancy (Gullan and Cranston 2000). We are therefore reporting mite activity as a function of calendar date rather than GDD (fig. 1A–1E). A bell-shaped pattern in mite activity was recorded at seven of 12 monitoring sites in 2013–2015 (fig. 1A–1C). In 2014, the Rutherford site (fig. 1B) recorded low mite activity compared with other sites but a small peak remained evident. We documented greater fluctuation in activity at the sites monitored in 2016 (fig. 1D and 1E), and we noted more than one peak in activity at several sites. At every site, ANOVA revealed that changes in mite activity were statistically significant at some point (ps < 0.05). Post hoc t-tests were employed to determine when mite activity differed significantly at the numerical peak from the rest of the season. This helped to identify the optimum window for sulfur applications that target migrating mites (table 2).
FIG. 2. Erineum mite activity on monitoring tapes was similar across shoot positions (basal, middle, upper) where tapes were deployed.
TABLE 2. Accumulated growing degree days (GDD) and calendar date for peak erineum mite activity and optimal timing of sulfur applications targeting migrating erineum mites
With few exceptions (fig. 1E; table 2), the optimal window for sulfur applications across all years was between Sept. 1 and Nov. 7 (2,199 to 3,859 GDD). At 10 of 12 sites, mite activity peaked numerically in October. For 11 of 12 sites, the optimal sulfur application period fell between the last week of September (after Sep. 21) and the first week of November (before Nov. 8). We therefore concluded that in the North Coast region, this six-week period is the best application window for sulfur treatments. This lengthy period should allow for some flexibility to delay applications until after the grape harvest. Because we recorded variability in mite activity across location and season, site-specific monitoring of the migration could determine peak activity and ensure that sulfur applications are responsibly applied. Monitoring practices could incorporate the use of double-sided tape, as demonstrated in this and other studies (Walton et al. 2007; Bernard et al. 2005), although further work is needed to optimize techniques for use by practitioners.
Sulfur applications reduced leaf blistering in the following season
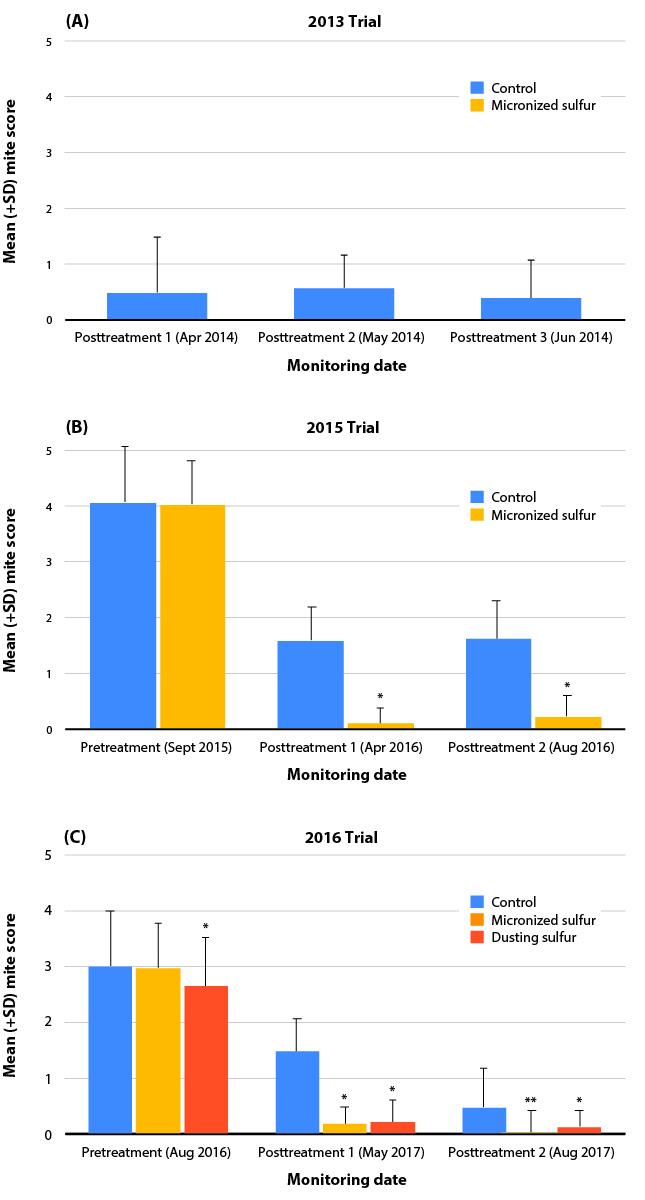
Across all trial sites and study years, a postharvest application of sulfur significantly reduced the incidence of leaf blistering in the subsequent growing season (fig. 3). In the 2013 trial (fig. 3A), no blisters were found on any treated vines — on any of the posttreatment monitoring dates (compared to the untreated vines (p < 0.001). In the 2015 trial (fig. 3B), there was no pretreatment difference in the incidence of leaf galling. However, there was a significant reduction in mean blister score on both the posttreatment monitoring dates, compared to the control (ps < 0.001) (fig. 3B). During posttreatment monitoring, mean blister scores in the control fell to 39% to 40% of the pretreatment score, whereas the dry flowable sulfur treatment reduced blistering to less than 10% of pretreatment values. In the 2016 trial (fig. 3C), there was a significant effect of sulfur treatment (p = <.001) but also a significant treatment x date interaction (p = < 0.001). Post hoc t-tests exploring the interaction revealed that pretreatment blister scores started lower (ps < 0.05) in the dusting sulfur blocks, compared with the control blocks. At posttreatment, both dry flowable and dusting sulfur blocks had significantly lower blister scores compared with the control blocks: Control scores were reduced to 49% and then 16% of pretreatment scores. Blistering in the dry flowable and dusting sulfur treatments were reduced to less than 10% of pretreatment incidence. By the second posttreatment monitoring, incidence of leaf blistering was significantly lower (p < 0.01) in the dry flowable compared to dusting sulfur treatment.
FIG. 3. Summary of mean blister scores for (A) 2013 trial site, (B) 2015 trial site and (C) 2016 trial site. In 2013 and 2015, a postharvest dry flowable sulfur application (5 lb per acre in 75 gallons of water) was compared to an untreated control. In 2016, sulfur treatments included both dry flowable sulfur (5 lb per acre in 75 gallons of water) and sulfur dust (12 lb per acre). An asterisk (∗) above the treatment bar indicates a significant effect of treatment compared to control. A double asterisk (∗∗) indicates significant effect of the dry flowable treatment compared to both control and dusting treatment.
These findings indicate that postharvest dry flowable sulfur applications in commercial vineyards can be used to reduce blistering associated with erineum mite. Dusting sulfur also appeared effective, though our evaluations were confounded by the low pretreatment mite populations in these blocks. However, in posttreatment sampling, blister formation in the dry flowable sulfur treatment was eventually lower than dusting sulfur, suggesting that the former may be more effective.
Conclusion
Postharvest applications of dry flowable sulfur significantly reduced the incidence of blistering of grapevine leaves by erineum mites in the subsequent growing season. Dusting sulfur may also be effective, although our results were confounded, suggesting that future studies should reassess the effects of this treatment. Future studies should also evaluate potential multiyear effects to determine application frequency, establish treatment thresholds and evaluate efficacy in other growing regions. Sulfur applications should be made during the period when mites are moving between their in-season feeding sites (leaves) to overwintering sites (buds). In our trial sites in Napa County, most of this activity occurred between the last week of September and the first week of November, indicating this as a key period to target applications aimed at reducing blistering. Variations across monitoring sites and seasons indicate the value of site-specific monitoring to elucidate local patterns of movement. The occurrence of the peak activity period should also be explored for other growing regions. Successful monitoring strategies will likely incorporate the use of double-sided tape, though these methods need to be optimized for uptake by practitioners. Postharvest sulfur applications are an alternative to in-season applications, particularly when leaf blistering is not obvious until later in the growing season (during berry ripening). CA
Data analysis
Migration patterns
Each vineyard was analyzed independently when testing for changes in mite activity over the monitoring period. Total (summed) mite activity was calculated for descriptive purposes to represent total number of mites found on each sampling date. Mean mite activity scores were then analyzed using a repeated measures ANOVA with tape scores as the dependent variable and time points (date) as the repeated measure variable. In each case this confirmed that mite activity varied at some point over the season. We then conducted selected post hoc paired t-tests between specific time points of interest to confirm when peak mite activity was significantly different from earlier and later in the season to justify when sulfur should be applied. A minority of time points were somewhat skewed (skewness statistic between 1 and 2), which transforming the data did not entirely correct. For this reason, we conducted exploratory non-parametric analyses using Friedman's Test and Wilcoxon Sign Tests, but the conclusions remained the same so in the main text we report results of the ANOVA and t-tests.
Shoot location
To generate sufficient data points at each shoot location (basal, middle, upper) to analyze effects of shoot location on mite activity, we examined collated mite activity across all monitored vineyards for each year.
General
Greenhouse-Geisser values were used where Sphericity was violated. In all statistical tests a p-value of 0.05 was taken as the criterion for significance, and where appropriate Holm's sequential Bonferonni adjustment was applied to post hoc tests.