Calag Archive
Calag Archive
Brown spot in table grape Redglobe controlled in study with sulfur dioxide and temperature treatments
Publication Information
California Agriculture 74(3):163-168. https://doi.org/10.3733/ca.2020a0022
Published online October 20, 2020
PDF | Citation | Permissions
NALT Keywords
Abstract
Brown spot is a postharvest disease of grapes caused by Cladosporium species in the San Joaquin Valley of California. It spreads during cold storage and transport, resulting in severe economic losses to late table grape cultivars, which are grown mainly for export to countries such as China and Mexico. We examined the effect of temperature and sulfur dioxide (SO2) treatments on fungal growth and infection of Redglobe berries by three Cladosporium species: Cladosporium ramotenellum, C. cladosporioides and C. limoniforme. Redglobe is especially popular for export. Fungal colonies growing on potato dextrose agar in petri plates stored at -2°C grew slower than those stored at 2°C, and an 400 ppm-h SO2 treatment significantly reduced fungal growth of all three species and at all temperatures tested. Redglobe berries inoculated with the Cladosporium species, treated with SO2 concentrations of 100 ppm-h, 200 ppm-h and 400 ppm-h and incubated in high relative humidity chambers for 28 to 32 days at 2°C, showed little incidence of disease. The development of brown spot on berries was entirely prevented with the treatment of 200 ppm-h SO2 for all Cladosporium species tested.
Full text
California is the leading producer of table grapes. In 2019 table grapes accounted for 130,000 acres of the 918,000 acres of grapes grown in the state, with 6,588 acres grown with the variety Redglobe (CDFA 2020). The cultivar Redglobe is a variety popular for export markets, including China and Mexico, because of its flavor and long shelf life (Dokoozlian et al. 1996). Brown spot can cause major postharvest fruit loss in Redglobe and other late-harvest cultivars such as Crimson Seedless and Autumn King (Swett et al. 2016). No reliable control of brown spot has been found.
A study by Swett et al. (2014) showed 100% of Redglobe clusters collected from a commercial field in Delano, in the San Joaquin Valley, had latent infections of Cladosporium species responsible for brown spot disease. Redglobe clusters may be stored for 2 to 3 months before they are shipped to Asia. When symptomless berries are in cold storage conditions for long periods, brown spot disease begins to emerge and spread (Swett et al. 2016). While initial infections occur in the field, once in post-harvest, infection can also easily spread from berry to berry through epidermis contact with no wounding necessary and in temperatures as low as −2°C (Swett et al. 2016). Brown spot is a major source of economic loss for grapes during long distance transport.
Harvested Redglobe table grapes. This variety is popular for export but can develop brown spot disease while in cold storage and transport. Laboratory test results indicate that sulfur dioxide treatments of at least 200 ppm-h can prevent the disease.
Attempts to manage brown spot have relied on strategies developed for the control of gray mold, a severe postharvest disease caused by the fungus Botrytis cinerea (Cantín et al. 2011; Coertze et al. 2001; Crisosto et al. 1994; Lee et al. 2015; Palou et al. 2001). Gray mold (Botrytis cinerea) and brown spot (Cladosporium spp.) have similar biology, such as infection timing, occurrence of a latency period and timing of symptoms expression (Coertze et al. 2001; Crisosto et al. 2002; Crisosto et al. 1994; Fourie 2008; Palou et al. 2001; Swett et al. 2014). However, the common practice of using 100 to 200 parts per million per hour (ppm-h) sulfur dioxide (SO2) treatments used to control gray mold during cold storage and during transport has not been effective for the control of brown spot (Lee et al. 2015; Palou et al. 2001; Serey et al. 2007; Smits et al. 2003; Teles et al. 2014).
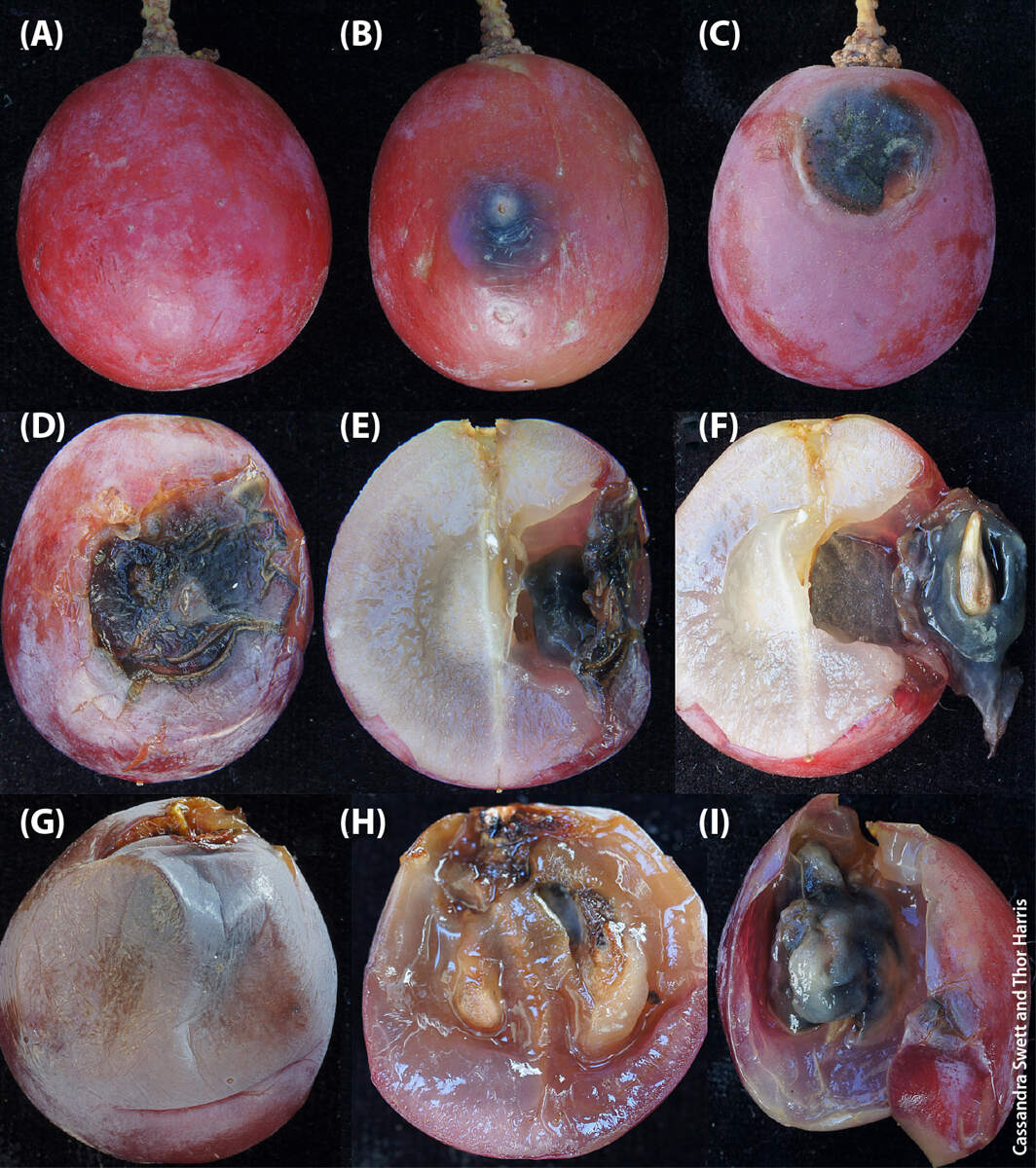
Brown spot has been attributed to several species of the Cladosporium herbarum species complex (C. ramotenellum, C. tenellum, C. limoniforme) and C. cladosporioides (Crous et al. 2007; Swett et al. 2016). As described by Swett et al. (fig. 1), typically a fluffy, light green to white mycelial mat will form where infection has taken place on the berry epidermis. A mycelial callus can form under the epidermis as a result of an internal infection, creating a scab and a brown spot on the underside of the epidermis. As the infection progresses, the scab will encompass the seed of the grape, forming a fungal fruiting body that eventually replaces the grape seed placenta, and prolific sporulation will occur on the seed (Swett et al. 2016; fig. 1).
FIG. 1. Brown spot postharvest on grape. (A) healthy berry, (B) early brown spot, (C) brown spot with cavity and fungal sporulation, (D) advanced brown spot, (E) fungal growth inside the berry, (F) fungus replacing the placenta and encasing the seed, (G) infected berry with no external spot, (H) spores lining the interior of the mycelial mat, (I) berry placenta replaced by fungal growth. Source: Swett et al. 2016.
In the last 20 years, a total utilization technique for fumigant applications of SO2 during cold storage has been established for table grapes; it increases efficiency, reduces environmental pollution and protects operators (Crisosto and Mitchell 2002; Luvisi et al. 1992;
Palou et al. 2002). An important step of the technique is to apply the first SO2 treatment (∼ 100 to 150 ppm-h) during the initial forced-air cooling of the grapes after harvest, which is then followed by weekly applications during cold storage with homogenous air distribution (Luvisi et al. 1992). The total utilization technique system uses ∼ 10 times less SO2 than the previous standard fumigation system, but it requires uniform room air distribution for the treatment to be effective (Crisosto and Mitchell 2002).
The total utilization technique was based on laboratory studies that revealed that at least 100 ppm-h SO2 was necessary to kill B. cinerea conidia and inactivate exposed mycelia at 0°C (Smilanick and Henson 1992). Even less than 100 ppm-h was effective at warm temperatures to cause the death of conidia and mycelium of B. cinerea on grape (Crisosto and Mitchell 2002; Luvisi et al. 1992). These latter studies confirmed that SO2 applied at 200 ppm-30 min, 400 ppm-15 min, 50 ppm-2 h or 25 ppm-4 h was as effective as the 100 ppm-h treatment (Crisosto et al. 2002; Palou et al. 2001, 2002). The sensitivity of B. cinerea conidia to SO2 increases two- to fourfold for every 10°C increment between 0 and 32°C, because of the effect of temperature on SO2 absorption on the fruit, fungi and surrounding packaging (Smilanick and Henson 1992).
SO2 has mainly been used to control gray mold disease of table grapes, but the low application rates applied as part of the total utilization technique have not prevented the emergence and spread of brown spot during cold storage (Fernández-Trujillo et al. 2012). The objectives of our two-part study were (1) to evaluate, in vitro, the effect of temperature and SO2 concentration over time applications on fungal colony growth of three Cladosporium species (C. ramotenellum, C. cladosporioides and C. limoniforme), and (2) to determine the efficacy of different SO2 concentrations over time in inhibiting the growth of Cladosporium species on artificially inoculated Redglobe berries.
Origin of fungal isolates
Cladosporium species (C. ramotenellum, C. cladosporioides and C. limoniforme) were isolated from brown spot–symptomatic berries grown in Delano, California, from 2013 to 2015. Species isolates were obtained from symptomatic tissue placed on 150 by 15 mm (millimeter) petri plates with 3% rose bengal potato dextrose agar (Difco Laboratories, Detroit, Mich.) amended with 500 ppm tetracycline and 300 ppm streptomycin.
Cladosporium colonies were hyphal tipped after 7 days to produce pure cultures for species identification. PCR (polymerase chain reaction) amplification of the actin gene using primers ACT-512 and ACT-783 and DNA sequencing confirmed the identity of species (Swett et al. 2016). Species isolates were maintained in Dr. W. Douglas Gubler's laboratory, Department of Plant Pathology, UC Davis, as of June 2016. Pathogenicity was demonstrated when species isolates were inoculated and re-isolated from lesions on Crimson Seedless berries following standard protocols (Swett et al. 2016).
Source, preparation of grapes
Redglobe berries were obtained from a commercial vineyard in Delano. Asymptomatic, nonwounded berries were removed from clusters with the pedicel still intact by clipping with sterile scissors. Berries were then vigorously washed in a 0.5% potassium chloride and 0.1% Tween 20 solution to remove surface debris. Berries were surface disinfected first in a 70% ethanol solution for 30 seconds, then in a 10% bleach solution for 5 minutes, and dried in a sterile laminar flow hood (Dugan et al. 2002).
Dry berries (n = 9 per fungal species tested) were aseptically distributed in triplicate on sterilized polyethylene chambers (215 by 300 by 105 mm) on sterilized polyethylene grids (210 by 297 mm) at 2°C with high relative humidity (RH 93%). RH was obtained with paper towels moistened with sterile deionized water and placed below a plastic grid. RH was measured with a humidity sensor (HOBO, Onset Computer Co., Bourne, Mass.).
Mycelial growth study
To determine the effect of temperature and SO2 on fungal growth, potato dextrose agar (PDA) plates were inoculated with one of three species: C. ramotenellum, C. cladosporioides or C. limoniforme. Inoculum was grown on 2% PDA at 23°C for 5 to 7 days. Plugs of colonized PDA obtained with a sterile 4-mm cork borer were placed on a 2% PDA plate, spore side down. The baseline for all measurements was 4 mm.
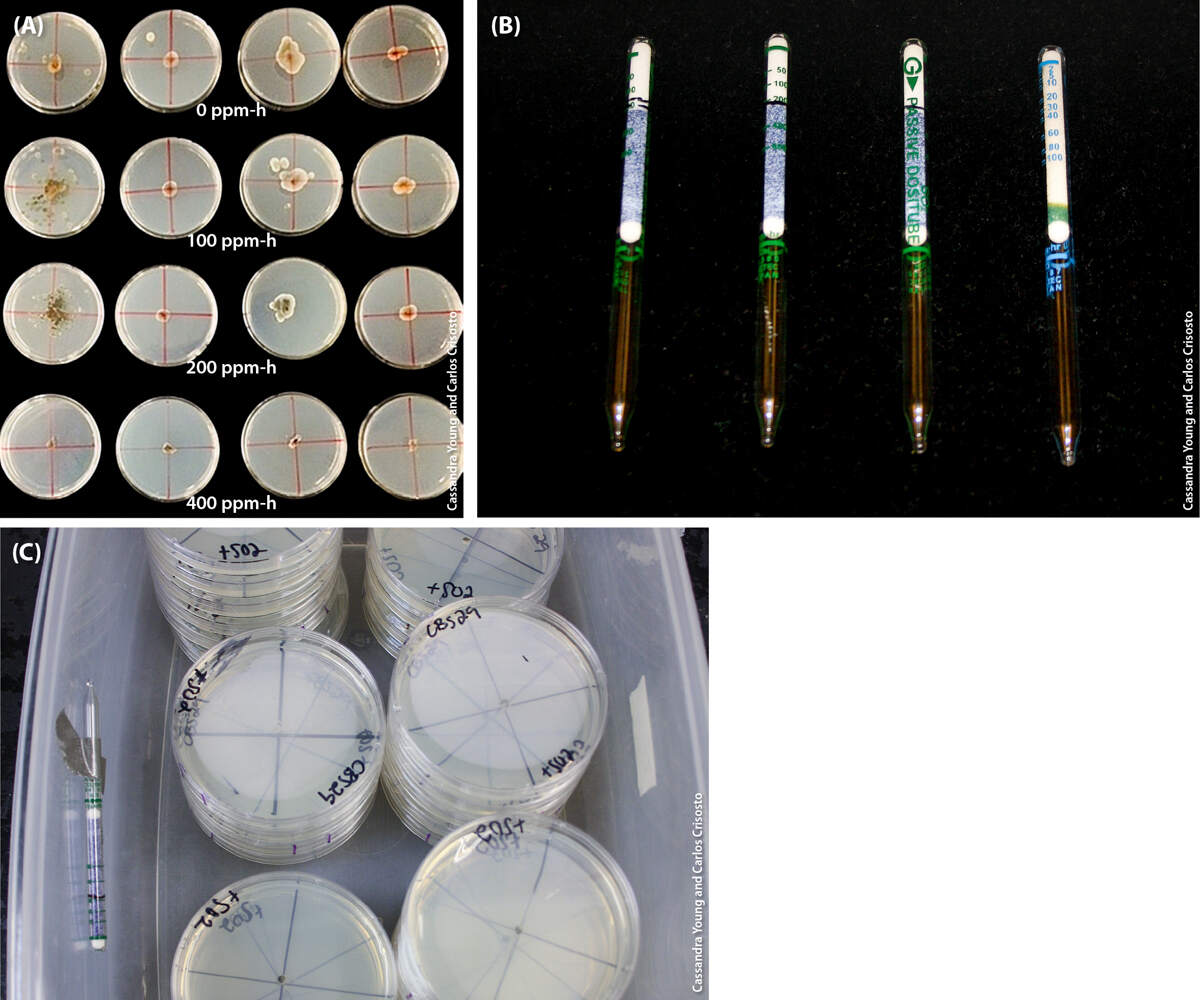
Inoculated plates were incubated in a polyethylene chamber at 23°C for 13 hours. The petri plates were moved to a fumigation chamber and exposed to the three SO2 treatments while still lidded. SO2 concentrations were measured using passive colorimetric dosimeter tubes (Gastec Corp., Ayase-Shi, Japan) and a portable SO2 detector that continuously measured SO2 concentration inside the fumigation chambers (Palou et al. 2002). Dosimeter tubes were taped to chamber walls opposite the SO2 flow as well as inside of a petri plate. Untreated controls were inoculated as previously described, received no SO2 exposure but were treated and incubated identically. For each species, three petri plates were inoculated and treated in triplicate and were incubated at 2°C and −2°C for up to 32 days after treatment.
Petri plates inoculated with one of three species — C. ramotenellum, C. cladosporioides or C. limoniforme — in a fumigation chamber and exposed to the three SO2 treatments while still lidded. SO2 concentrations were measured using passive colorimetric dosimeter tubes and a portable SO2 detector.
Radial measurements of fungal colonies were taken to assess the effect of SO2 concentration over time on mycelial growth. Colony measurements were taken from the reverse side of the lidded petri plate. Using a polyethylene ruler, two perpendicular diameter measurements were made and averaged to determine overall colony size. The diameter of the mycelium plug inoculum (4 mm) was included as the minimal measurement for all treatments. Measurements were taken every 6 to 8 days during incubation at 2°C and −2°C.
Inoculated berry study
Inoculated berries were not wounded prior to inoculation. Inoculum was prepared with 14-day-old cultures grown on PDA. Spores were suspended in 0.5% potassium chloride and 0.1% Tween 20 in sterile distilled water. Spore density was determined using a hemo-cytometer, and the suspension was adjusted to 1 × 107 spores/mL by the addition of sterile deionized water.
The shoulder of each berry was inoculated by placing 10 μL of spore suspension within a 4-mm Vaseline ring, to prevent the inoculum from moving. Berries were positioned with the inoculated shoulder facing up. Untreated controls consisting of berries inoculated with sterile deionized water were included for each temperature and SO2 treatment. Berry lesions on un-wounded berries were measured after 28 days of storage at 2°C.
Concentration-time SO2 treatments
Twenty-four hours after berries were inoculated with one of the three Cladosporium species, they were exposed to three concentration-over-time treatments of gaseous SO2: 100 ppm-h (100 μL/L per h), 200 ppm-h (100 μL/L per 0.5 h) or 400 ppm-h (100 μL/L per 0.25 h) (Palou et al. 2001). SO2 concentrations were measured as previously mentioned. Dosimeter tubes were taped to chamber walls opposite the SO2 flow.
For both studies, fumigation chambers were Sterilite containers (Sterilite, Townsend, Mass.) modified to allow for a rubber tube to flow SO2 into the chamber. Chambers were placed inside a biological safety cabinet during treatment. Chamber lids were opened once a treatment was concluded and the inoculated berries on a grid/mycelial plugs on petri plates were placed into polyethylene chamber to be stored in cold storage.
Statistical analysis
Radial growth of Cladosporium colonies was analyzed using a linear mixed model approach for all three species using the R package lme4 (Bates et al. 2014). The mixed model used a Gaussian error distribution and consisted of SO2 treatments, temperature and species as fixed effects and replicate as a random effect. We calculated the estimated marginal means and computed all pairwise comparisons using Tukey's honestly significant difference (HSD) test.
For the berry study, we analyzed the proportion of infected berries with a linear mixed model approach using a Gaussian error distribution for all species, with SO2 treatments and species as fixed effects and replicate as a random effect and computed all pairwise comparisons using Tukey's HSD test.
Fungal growth significantly reduced
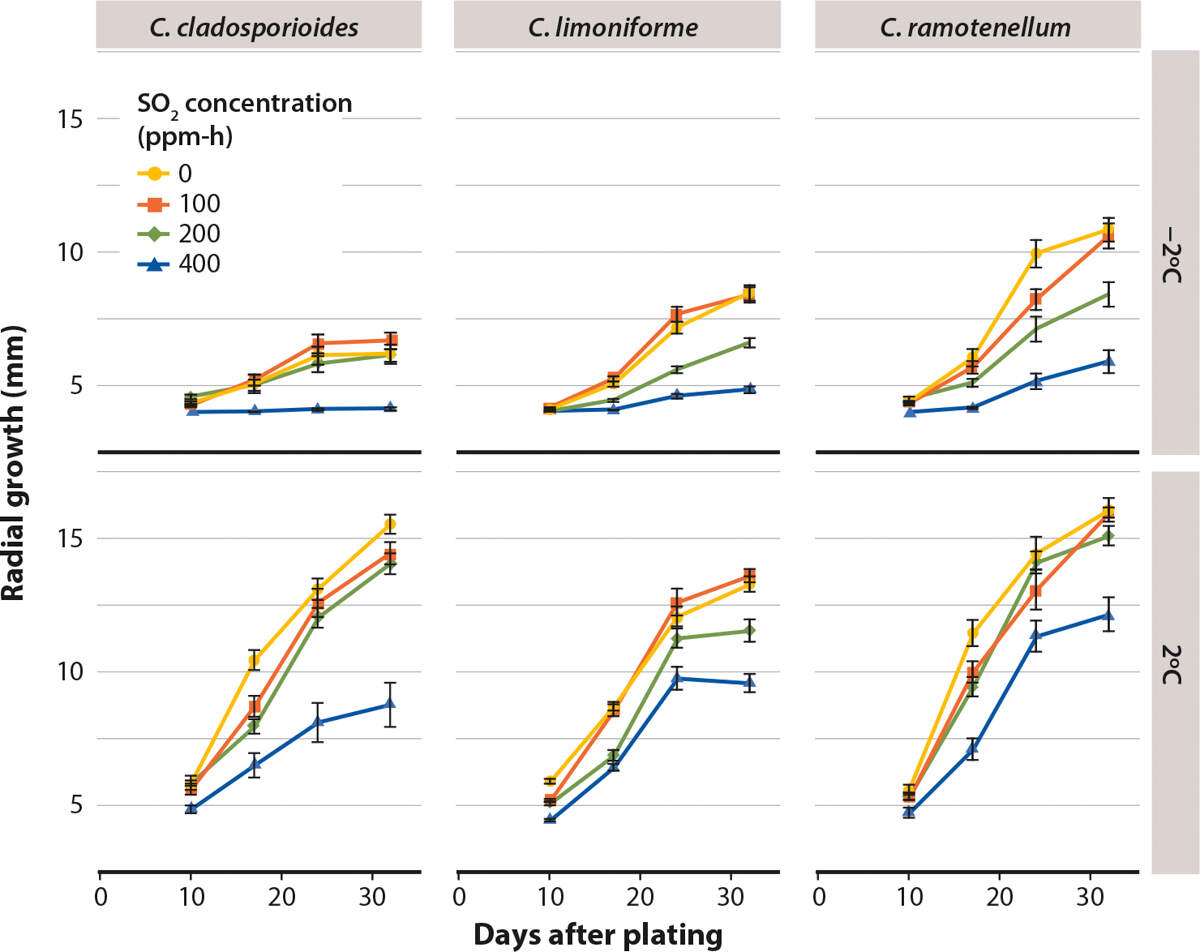
Radial colony growth of the three Cladosporium species on petri plates with PDA was significantly reduced by the 400 ppm-h SO2 treatment, as seen in figures 2 and 3. The 400 ppm-h concentration was most effective against C. cladosporioides when petri plates were incubated at −2°C, which resulted in no radial growth. The 400 ppm-h was also effective in slowing C. cladosporioides colony growth at 2°C; radial growth grew only from 4 to 9 mm on average.
FIG. 2. (A) Mycelial growth of C. cladosporioides, C. limoniforme and C. ramotenellum in lidded petri plates treated with SO2 of 0, 100, 200 or 400 ppm-h. (B) Passive colorimetric dosimeter tubes were used to measure SO2 concentrations. (C) One was placed inside each fumigation chamber, opposite the SO2 input valve.
FIG. 3. Influence of 0, 100, 200 and 400 ppm-h SO2 on radial growth of three Cladosporium species plated on PDA growth medium using a 4-mm mycelial plug inoculum. Lidded petri plates were incubated in cold storage at −2°C and 2°C for up to 32 days. Mean ± standard error.
Radial colony growth of C. limoniforme and C. ramotenellum was also slowed down at 400 ppm-h, growing from 4 to 5 mm and 4 to 6 mm, respectively, at −2°C. However, the same treatment was less effective at 2°C, where, on average, fungal radial growth reached 10 mm for C. limoniforme and 12 mm for C. ramotenellum after 30 days. At the lower SO2 ppm-h (200 ppm-h, 100 ppm-h), there was no significant difference in radial colony growth between any of the treated species and the untreated controls by 30 days at 2°C.
All species after 10 days at −2°C had reduced colony growth for all concentrations compared with those incubated at 2°C. The colony growth of C. cladosporioides at −2°C was slower than that of the other species; and it was also slower than the colony growth of C. cladosporioides incubated at 2°C, illustrating the effect of temperature on rate of growth.
200 ppm-h treatment eliminated disease
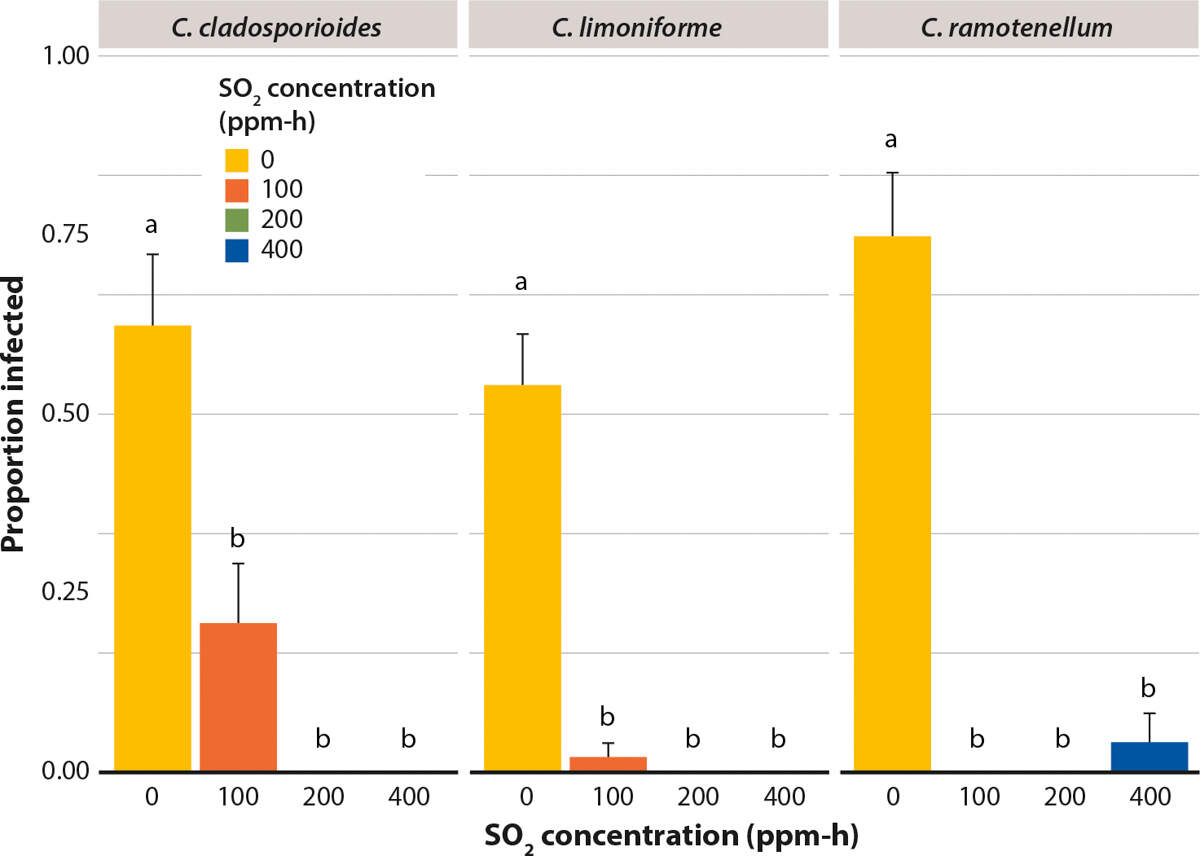
In the berry study, all Cladosporium species caused disease in untreated control berries: C. cladosporioides caused disease in 65% of berries, C. limoniforme in 55% berries and C. ramotenellum in 75% of berries (fig. 4). By contrast, SO2 treatments significantly reduced disease incidence by Cladosporium species on inoculated Redglobe berries.
FIG. 4. Influence of SO2 at three concentrations on the proportion of infected Redglobe berries after inoculation with three species of Cladosporium. After inoculation, berries were placed in cold storage at 2°C, RH 93%. Lesions on berries were measured at 28 days. Mean ± standard error. Letters represent significant difference at α = 0.05, according to Tukey's honestly significant difference (HSD) test.
The 100 ppm-h treatment reduced disease incidence to less than 25% of berries for all species. The 200 ppm-h treatment was the most effective, in that it eliminated disease for all three Cladosporium species tested. In the 400 ppm-h treatment, there was less than 5% disease incidence in berries inoculated with C. ramotenellum and no infection occurred in berries inoculated with C. cladosporioides or C. limoniforme. Nevertheless, there was no statistically significant difference in the proportion of infected berries between the 100, 200 and 400 ppm-h SO2 treatments of the three Cladosporium species tested (fig. 4).
Potential of SO2 as a control for brown spot
Our results demonstrate that a single application before cold storage of at least 200 ppm-h SO2 at cold storage temperatures may be an effective tool for reducing brown spot disease incidence on grape berries, although the ability of mycelium of Cladosporium species to grow on PDA growth medium in petri plates with an SO2 application was somewhat variable. We have demonstrated SO2 of 200 ppm-h and 400 ppm-h essentially prevent disease development in Redglobe berries from the three Cladosporium species tested. C. cladosporioides exhibited tolerance at 100 ppm-h, whereas 100 ppm-h was effective against C. ramotenellum and C. limoniforme. While mycelial growth on PDA was reduced with a treatment of 400 ppm-h SO2, mycelial growth was not completely eliminated. Additionally, C. ramotenellum grows faster than the other species on a petri plate with PDA, regardless of temperature or SO2 treatment. Unsurprisingly, in the berry study, inoculation of C. ramotenellum resulted in the highest disease incidence in the untreated control berries. Our study also confirmed the critical importance of maintaining table grapes below 0°C and above their freezing point to maximize their storage life potential (Crisosto and Mitchell 2002).
These results are promising for the control of brown spot during long-term storage and export of Redglobe table grapes. Although not much is known about the natural berry infection by Cladosporium, we do know that infection can occur on the surface through the epidermis and may also be a latent infection where infection starts from within the berry. Berries used in our experiments were surface sterilized before inoculation so we could be sure that infection occurred through the epidermis with our inoculum; thus, we can conclude the SO2 application prevented disease from of the Cladosporium surface inoculum. However, SO2 may not be adequate to prevent infection resulting from latent infection, causing internal rot of the berry and inoculum production, but may inhibit the resulting spread if applied after the emergence of fungal growth (Harvey 1955; Lee et al. 2015; Swett et al. 2016).
This work demonstrates a potential management strategy of brown spot post-harvest. In practice, multiple factors can influence the amount of gaseous SO2 that comes into contact with the berry surface, including air flow in storage, sorption of packing materials and the amount of condensation on berries, which can absorb gasses. Additionally, a single application of SO2 is typically not as effective as multiple SO2 applications, but multiple applications may not be feasible during transport (Crisosto et al. 2002). Total utilization technique focused on gray mold control applies SO2 at 100 to 150 ppm-h, which may be too low to be effective against brown spot, especially under cold storage conditions. Future studies should be done to improve sampling and the detection of brown spot in the field prior to harvest as well as during postharvest, so this research can be applied to known compromised lots in transport and better determine the efficacy of this practice under commercial conditions.