Calag Archive
Calag Archive
Phylloxera on rise…: Deadly insect pest poses increased risk to north coast vineyards
Publication Information
California Agriculture 45(2):30-32.
Published March 01, 1991
PDF | Citation | Permissions
Abstract
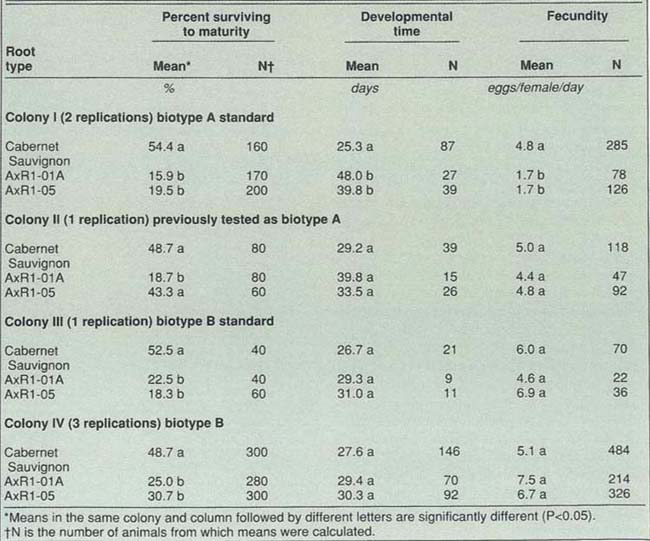
Resistant rootstocks protect grape vines from phylloxera; however, a new form of this insect, Biotype B, threatens the survival of 70% of Napa and Sonoma County vineyards, those which are planted on the rootstock AxR#1. Research demonstrates that different accessions of AxR#l are equally susceptible to damage by this insect, a form of plant lice. The insect has spread from two sites in 1983 to more than 70 sites in those two counties; spread to other grapegrowing counties is likely.
Full text
Grape phylloxera is a widespread pest of grapevines. Native to eastern North America, it was introduced into Europe in the 1860s where it devastated vineyards. In response, European researchers developed resistant rootstocks. Many rootstocks available today are a result of breeding and selections made in Europe during the last quarter of the 19th century. One of these, Ganzin 1, known in California as AxR#1, was initially successful. However, in both Europe and South Africa, resistance disappeared after a number of years. The loss of resistance was attributed to a more virulent race of phylloxera.
Phylloxera became an important problem in California with the introduction of the European grape, Vitis vinifera. Trials to determine which rootstocks were suited to various California conditions were established by Husmann in 1905 and by Jacobs after 1929, the repeal of Prohibition. Jacobs' trials were continued by Lider in the 1950s, and in 1958, Lider concluded that the root-stock AxR#l, a hybrid of V. vinifera and V. rupestris, was sufficiently resistant to phylloxera in California and was suited to a variety of growing conditions in the state, particularly those in the coastal valleys. As a result, AxR#l usage increased markedly, and by the late 1970s, this rootstock predominated new plantings in northern California.
Severe damage to AxR#1 by phylloxera was first identified in California in 1983 at two sites in Napa and Sonoma Counties. Since then, the biotype has spread to more than 70 sites in those two counties. Laboratory and field tests established that a different strain of phylloxera was responsible for the damage. The new strain was named biotype B to distinguish it from the then more common strain, biotype A, which does not damage AxR#1. These tests determined that a number of the currently available root stocks were resistant or immune to the new biotype (see California Agriculture, January-February, 1987).
The current study was undertaken to determine if there was any difference in the resistance of two AxR#1 accessions distributed by the University of California's Foundation Plant Material Service. The two accessions, AxR#1-01A and AxR#1-05, exhibited structural differences in leaves and shoots, so it seemed possible that resistance to biotype B might also vary. If so, the solution to the problem would be to restrict planting to the more resistant AxR#1 clone.
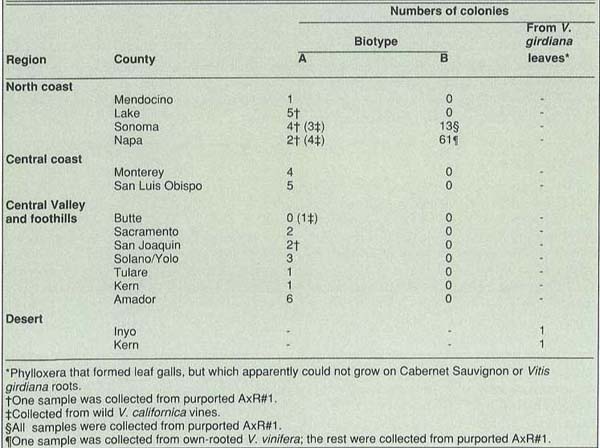
TABLE 2. Determinations of grape phylloxera biotypes. Collections mere (mm own-rooted, Vitis vinifera vines except as noted.
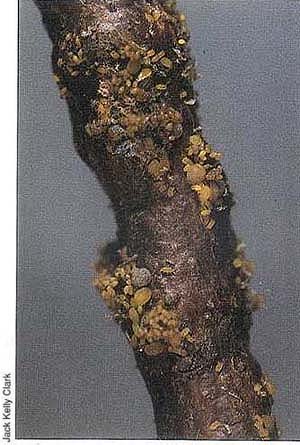
Above; Leaf gaits of grape phylloxera, such as these on the wild grape Vitis girdiana of southern California, are unknown on cultivated grapes in this state.
Right: Feeding by dense aggregations of phylloxera adults, nymphs and eggs causes root swelling and eventual death.
In addition, we surveyed known phylloxera sites to determine how widely type B had spread from the two original sites. Knowledge of proximity is needed by vineyardists for their planning and management decisions. We also sampled noncultivated native grapes and hybrids for phylloxera.
Methods and Results
Resistance of AxR#1 accessions
The two accessions tested were AxR#1-01 A and AxR#1-05 from two nurseries in Yolo County. Cabernet Sauvignon roots were used as controls because they are highly susceptible to A and B biotypes of phylloxera. We obtained phylloxera from four laboratory colonies: the two colonies that are our standards of biotypes A and B, one which previously tested as biotype A, and another biotype B colony.
We placed phylloxera eggs on root pieces and determined percentage surviving, development time for eggs to become adults, and number of eggs laid per day for the first 10 days of adult life. In seven replications, we placed 40-100 eggs on each of three root types, 10 eggs per root piece. We used only data on phylloxera which established on the main portion of the roots.
Values on the two AxR#1 accessions were compared for each colony using t-tests at 95% confidence (table 1). There were no significant differences between the two accessions in 11 out of the 12 statistical comparisons. The exception, a greater percentage of colony II individuals surviving to maturity on AxR#l-05 than on AxR#1-01 A, likely was due to random variation.
Both accessions are susceptible to biotype B, but maintain resistance to biotype A (see table 1). Overall, the lack of significant differences between AxR#1 accessions indicates that the failure to resist phylloxera cannot be attributed to the choice of one certified AxR#1 accession over the other.
The bioassays also confirmed biotype differences with regard to developmental times and fecundity. Here again, colony II was anomalous; it responded as an A colony previously and subsequently, but in this experiment responded inexplicably as a B colony.
Survey
Sites for collection of phylloxera were chosen based on knowledge of infestations from 15 counties (table 2). Samples from Napa and Sonoma County vineyards were primarily from AxR#1; most samples from vineyards in other counties were from own-rooted Vitis vinifera vines. Phylloxera were also collected from non-cultivated Vitis californica and vines that appeared morphologically to be V. californica x V. vinifera hybrids, and, in the desert region, from V. girdiana in Walker Canyon near Inyokern and Scotty's Castle in Death Valley. All collections were from roots except for samples from V. girdiana, which came from leaves. We successfully established about 70 % of all collections on root pieces as separate laboratory colonies which later underwent laboratory bioassays to determine biotypes.
Left: Phylloxera damage spreads radially creating an irregular patch of decline with the most severely stressed vines (just beyond the foreground) at its center.
None of the collections of phylloxera from leaf galls from V. girdiana colonized Cabernet Sauvignon or V. girdiana roots in the laboratory, and we did not find root-feeding forms on wild V. girdiana in the field at the times of leaf collections in October 1989 and April 1990. Roots of the same plants from which infested leaves were taken could be colonized by biotype A and B phylloxera in the laboratory. We do not know if the apparent differences between the leaf-feeding phylloxera and the root-feeding biotypes indicate that the former are of a different biotype or species, or if root feeding is possible at other times in the year.
Results of biotype determinations are shown in table 2. Biotype B phylloxera is widespread in Napa and Sonoma Counties. Although it is unknown elsewhere at present, we believe it will continue to spread by natural means (wind) and human intervention (on rootings, machinery, boxes, etc.).
Biotype B has not been found on roots of mature, own-rooted V. vinifera or V. califomica except once, in Napa County on V. vinifera. The reason for this is not yet known.
At sites where biotype B is found, growth and production by AxR#1-rooted vines are severely depressed and many vines are being removed. The increase in biotype B sites and severity of the vine depression suggest that long-term survival of AxR#1 is not likely, especially if contiguous with viticultural areas where the biotype B phylloxera has been found.
Discussion
Based on the early results of this study, the University of California recommended discontinuing the use of AxR#1 in December 1989. Because we expect the spread of biotype B to continue, the recommendation applies statewide.
Replanting of biotype B-infested vineyards with rootstocks that are resistant to this strain is already beginning. A number of rootstocks are known to be resistant to biotype B, however, because of other viticultural characteristics, no single rootstock is likely to dominate new plantings as did AxR#1. Necessary high-priority research for the future will include rootstock breeding and trials.
CORRECTIONS:
Due to editorial error, the January-February issue of California Agriculture carried two incorrect captions:
The significance of phylloxera on wild grapes is not known. They may harbor only certain biotypes, and populations on V. californica may be a reservoir from which phylloxera spread to vineyards. Phylloxera from V. girdiana differ from other populations in California but may be more similar to phylloxera populations we observed on leaves of native V. arizonica collected near Tuscon, Arizona and Caliente, Nevada. All may be native and of a different biotype or species.