Calag Archive
Calag Archive
In outdoor planters in commercial centers… Insect-parasitic nematodes are effective against black vine weevil
Publication Information
California Agriculture 47(3):16-18.
Published May 01, 1993
PDF | Citation | Permissions
Abstract
Insect-parasitic nematodes suppressed black vine weevil larvae in planters containing ivy vines at a commercial building. After 1 year, weevil numbers were lower in containers treated with nematodes either with or without an added alternate host.
Full text
Above: Containerized landscape planting of kangaroo ivy in a commercial complex is infested with black vine weevil. At left: Healthy larva and pupa are seen, left;steinernematid-infected black vine weevil larva and two pupae are right.
The black vine weevil (BVW), Otiorhynchus sulcatus, has long been recognized as a serious pest of container-grown nursery plants, ornamentals in landscapes and field vine crops. Its larvae cause damage by feeding on roots and girdling stems at the soil surface. This univoltine species (having one generation per year) and its associated feeding damage have been described previously in California Agriculture (March-April 1984, January-February 1985, and May-June 1989).
Briefly, the flightless, parthenogenetic females (males are unknown in this species) emerge in early spring and feed at night on foliage, characteristically leaving small notches along leaf margins. From 20 to 30 days after emergence, the females start depositing eggs at the soil surface near plants. Each female can produce up to 500 eggs. Hatching occurs within 10 days at soil temperatures of 24°C (75°F). The white, legless larvae feed on the roots; under high population densities they girdle plants at the soil surface, killing them. BVW larvae overwinter in the soil until pupation occurs in spring.
Many of the plants beautifying shopping centers and commercial buildings are favored hosts for BVW. A partial list of plants susceptible to BVW includes azaleas, cyclamens, escallonias, euonomyous, grapes, ivy, liquidamber, junipers, and rhododendrons. Because all BVWs are female, infestation can start with one adult and spread throughout plantings or by the moving of infested plants or soil containing eggs, larvae or adults. The nocturnal habit of BVW adults and the location of larvae and pupae in soil make detection difficult. Once established, an infestation may go undetected for several years and may only be noticed when plants start to show dieback.
BVW infestations in nurseries, outdoor landscapes and field vine crops can be suppressed, often with chemical insecticides, but in commercial areas catering to shoppers and outdoor diners and surrounded by containerized plants, chemical control is difficult. Biological control, therefore, is an attractive alternative. Alerted to an infestation in outdoor containerized plants within a commercial complex in San Francisco's Embarcadero, we initiated a year-long biological control program against BVW larvae and pupae, using insect-parasitic nematodes.
Experiment background
BVW larvae and pupae are highly susceptible to insect-parasitic nematodes in the families Steinernematidae and Heterorhabditidae. These nematodes, most useful against many soil insect pests, possess many attributes of successful biological control agents, including their ability to actively seek hosts, their availability from commercial sources, the safety in which they can be used among nontarget organisms including humans and their ease in application. Free-living, infective-stage nematodes, applied to the soil for insect control, seek and penetrate a suitable insect host, causing death within 48 hours. The nematodes complete their life cycle within the insect cadaver.
Previously, we had established that without an insect host in the soil, the infective-stage nematode did not survive for more than 50 days. Because we were unable to assess the initial BVW population densities in the containers, we incorporated a nonplant feeding (nonphytophagous) alternate host to enhance the nematodes' ability to produce viable progeny and to survive. Our objective was to establish these insect-parasitic nematodes as long-term control agents of BVW.
Experiment's design
Our approach was to enhance the reproduction of these insect-parasitic nematodes (and their long-term control of BVW) in soil planted with kangaroo ivy (Cissus antarctica) vines. Fiberglass planters, 18 x 61 x 23 cm (7 x 24 x 9 inches) deep, with three drainage holes in the bottom, contained three ivy vines and a commercial potting soil of 79% sand, 17% silt and 4% clay. The soil consisted of 25.5% organic matter and had a pH of 4.7 and an electrical conductivity of 5.6. To determine whether the presence of an alternate insect host would bolster nematode persistence and BVW control, half of the treatments received 10 mature Galleria mellonella larvae. These nonfeeding, nonphytophagous larvae, which are highly susceptible to the nematodes, were buried about 2.5 cm (1 inch) deep and spaced equally around the perimeter of each planter.
The nematodes used in the experiments, Steinernema feltiae (= bibionis) FN strain and Heterorhabditis bacteriophora NC1 strain, were obtained commercially from biosys (Palo Alto, California) and Bioenterprises (Roseville, Australia), respectively. There were six treatments: (1) with Galleria, (2) without Galleria, (3) with S. feltiae and Galleria, (4) with S. feltiae and without Galleria, (5) with H. bacteriophora and Galleria, and (6) with H. bacteriophora and without Galleria. Each treatment was replicated three times with four planters per replicate. Plants were watered, fertilized, and maintained by grounds personnel at the center.
Steinernema feltiae and H. bacteriophora were applied at a rate of 50 nematodes/cm2 and 74 nematodes/cm2 (327 and 474/inch2), respectively. Nematodes were applied with a hand-held, 8-liter (2-gal) Hudson sprayer on February 3, 1989 between 6 and 8 a.m. The alternate host was placed into the soil before the application and again on May 26, 1989. Before treatment, each planter's soil was bioassayed to determine the presence of naturally occurring nematodes. Three soil subsamples were taken (about 17 cm3 for each subsample from around the base of each ivy vine within a planter), placed into a 250-cc (9-oz) plastic cup, transported to the laboratory, and baited with six Galleria larvae that served as the bioassay insect to detect the presence of the insect-parasitic nematodes. The cups were capped and stored at room temperature. One week later, the bioassay insects were removed from the cups, and the dead larvae dissected and examined for the presence of nematodes. Using this same bioassay technique at prescribed intervals (2 to 3 weeks) after treatment, the soil from each planter was sampled separately for nematode persistence. Nematode persistence in the soil was measured by the percentage of the bioassay insects infected with nematodes. All soils were steam-sterilized and returned to planters after each sampling. At the end of the experiment on February 15, 1990, all planters were destructively sampled by removing the soil and counting the number of living BVW larvae and pupae.
Results
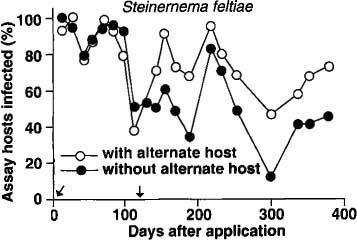
No insect-parasitic nematodes were found in any planters before nematodes were added or from control planters with or without the addition of the alternate host after treatment. In the S. feltiae plots, there was no difference in nematode persistence between planters with or without the alternate host for the first 100 days after application (fig. 1). However, after the second addition of the alternate host (112 days after nematode application), the percentage of bioassay insects infected with nematodes was usually higher in the planters with the alternate host than in those without the alternate host.
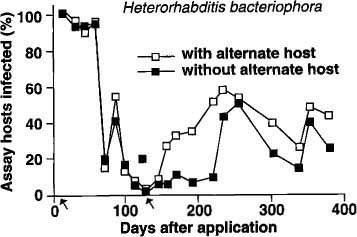
Similar trends were observed with H. bacteriophora, although at a lower level of nematode infection compared with S. feltiae (fig. 2). There was no difference in nematode persistence among planters with or without the alternate host for the first 70 days. After this period, the percentage of H. bacteriophora-infected bioassay insects dropped drastically, rebounding slightly between 80 and 100 days, and then dropping to less than 10% between 100 and 140 days. In planters with the second addition of the alternate host, there was a marked increase in bioassay insects infected with the nematode from 160 days onward. Planters without the alternate host remained at a low level (less than 15% bioassay insects infected) until 240 days when the percentage of infected bioassay insects increased to 50%.
Fig. 1. Mean percentage of nematode-infected bioassay insects (Galleria) recovered in 1 year from soil treated with Steinernema feltiae. (Arrows indicate times when the alternate host was added to planters.)
Fig. 2. Mean percentage of nematode-infected bioassay insects (Galleria) recovered in 1 year from soil treated with Heterorhabditis bacteriophora. (Arrows indicate times when the alternate host was added to planters.)
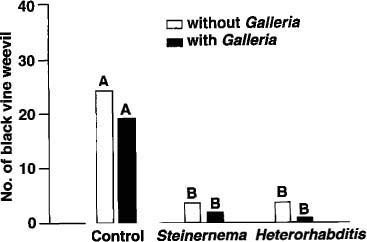
Fig. 3. The mean number of black vine weevils per planter in control, Steinernema feltiae, and Heterorhabditis bacteriophora treatments with and without the addition of an alternate host. Bars with the same letter are not significantly different at the 5% level.
Both S. feltiae and H. bacteriophora treatments with the alternate host had a higher nematode population than treatments without the alternate host, suggesting that the nematodes used them to reproduce. Yet, the long persistence of S. feltiae and H. bacteriophora in treatments without the alternate host suggested that they, too, were reproducing in BVW larvae and pupae. Indeed, upon destructive sampling of all planters at the end of the experiment, BVW larvae and pupae were recovered from all treatments (fig. 3). Control planters had significantly higher BVW populations than did the nematode treatments, but no significant differences were observed among nematode treatments with or without the alternate host.
Conclusions
Our experiment demonstrated that S. feltiae and H. bacteriophora can reproduce when suitable hosts are present in potted soil. More significantly, the nematodes reduced BVW infestations to a very low level. The periodic addition of alternate hosts to boost nematode populations is not practical for these commercial buildings where large numbers of planters are maintained for several years. Rather, a periodic check for BVW as previously suggested (California Agriculture, January-February 1985) may be a more prudent method for managing this insect. If BVW are found, nematodes can be applied and then augmented, as needed, to bolster the existing nematode population in suppressing this pest. Because they are safe to use and their potential for long-term control is evident, insect-parasitic nematodes are preferable to chemical control in managing BVW in buildings with containerized landscapes.