Calag Archive
Calag Archive
Why lacewings may fail to suppress aphids … Predators that eat other predators disrupt cotton aphid control
Publication Information
California Agriculture 47(5):7-9.
Published September 01, 1993
PDF | Citation | Permissions
Abstract
Biological control of the cotton aphid involves complex interactions among predators now under study.
Full text
Rising costs of insecticides, widespread insecticide resistance and increasing restrictions on insecticide use in California have spurred interest in insect management by other means, including biological control. Generalist insect predators are frequently abundant in annual crops, including field and vegetable crops, and have been identified as important in suppressing populations of damaging insects. However, effective use of such natural enemies to manage pests requires more complete understanding of insect ecology, including the biology of insect predators.
In cotton, for instance, generalist predators may be critical in controlling populations of spider mites, Lygus bugs and several worm pests. Few pest management experts or insect ecologists have studied interactions between insect predators, leaving the impression that insect predators feed exclusively on herbivorous arthropods. However, it is possible in theory that some generalist predators may attack other predators, with potentially negative effects on pest control. Here, we report a study designed to determine the effectiveness of lacewing larvae, Chrysoperla carnea, as biological control agents of the cotton aphid, Aphis gossypii, which feed on mid- and late-season cotton in the San Joaquin Valley. We found that a number of generalist predators impose heavy mortality on lacewing populations and thereby render natural and augmented populations of lacewings ineffective as biological control agents.
The cotton aphid, a major pest of cotton in California, presents very different problems for cotton production at different times of the year. Early season populations, which develop on very small cotton seedlings (often on plants with fewer than six nodes), can cause crinkled leaves, partial defoliation and stunted plant growth. Nevertheless, research still in progress suggests that this damage is fully compensated for before harvest and that the timing of crop maturation, quantity of yield and cotton fiber quality are unaffected. At mid-season, cotton aphid populations are frequently low; however, during 1992, populations in the southern San Joaquin Valley grew rapidly during July and August, and yield losses were apparent. As has been found in studies of several different cotton insect pests, plants that are setting bolls appear to have limited abilities to compensate for feeding damage. During the late season, when bolls are opening and cotton lint is exposed, cotton aphids create problems by excreting large quantities of sugary honeydew, which fall onto lint and create “sticky cotton.” Problems with sticky cotton become apparent during harvest, ginning and yarn manufacturing, and threaten overseas markets and the price premiums California cotton has historically received. Because the cotton aphid is already resistant to many insecticides in California and an even larger array of pesticides in the southern United States, long-term management of aphids will probably need to rely on noninsecticidal alternatives.
Cotton grown in the San Joaquin Valley generally develops large populations of generalist predators, including big-eyed bugs (Geocoris spp.), damsel bugs (Nabis spp.), assassin bugs (Zelus spp. and others), minute pirate bugs (Orius tristicolor) and lacewings (primarily Chrysoperla carnea). Other predators, less abundant, are also present. Lacewing larvae, known as potential predators of aphids in many crops, are available from several commercial insectaries. Some growers use augmentative releases of purchased lacewings at the recommended rate of 5,000 eggs per acre to improve control of cotton aphids.
Natural densities of lacewings
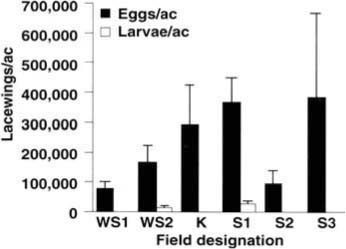
The first question addressed was: How many lacewing eggs and larvae are naturally present in late-season cotton fields when sticky cotton is a potential problem? Six fields were sampled between August 26 and September 2, 1992. In each field, entire plants were sampled and carefully inspected to count all lacewings present. The number of plants per row meter was recorded to translate counts into lacewings per acre. As shown in figure 1, natural densities of lacewing eggs were in all cases high, ranging from 77,000 to 380,000 per acre. Clearly, these naturally present eggs were so abundant in the sampled fields that more releases of insectary-reared lacewing eggs at the recommended rate (5,000 per acre) would not be useful. Observations of many other fields suggest that lacewings establish large densities of eggs in cotton fields at midseason and that these densities remain fairly stable for nearly all of the remainder of the growing season. Thus, before releasing lacewings, sampling of naturally present lacewings is recommended to determine whether releases will substantially increase densities in the field.
Adult assassin bug, Zelus renardii. Assassin bugs were found to be major predators of lacewing larvae.
Although egg densities were uniformly high, in four of the six fields we recovered no lacewing larvae (fig. 1), despite the fact that in several of the fields cotton aphid prey were abundant. This suggested that some factor, other than food limitation, was causing heavy mortality of young lacewing larvae.
Lacewing egg releases
Methods
Experiments to assess the effect of lacewing egg releases were conducted at the Kearney Agricultural Center and the UC Cotton Research Station at Shafter in 1991. Two treatments, the release of 5,000 insectary-reared lacewing eggs and no eggs released, were replicated ten times in a randomized block design. Our release rate, about 260 times the recommended rate of 5,000 eggs per acre, was chosen to determine whether any effect from lacewings could be discerned; smaller releases would have been dwarfed by naturally present populations. Releases at these rates are not, however, commercially feasible. Each plot was 10 feet by 6 rows of cotton, cultivar GC-510. Two days before the lacewings were released and weekly thereafter, aphid densities in each plot were quantified by sampling ten leaves per plot and counting all aphids. To determine the impact of the lacewings, we used the ratio of postrelease aphid counts to prerelease aphid counts.
Results
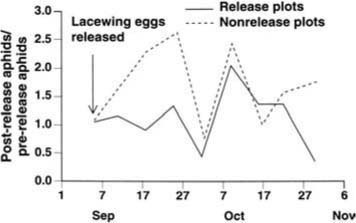
Because the effects of the lacewing releases were similar in the two experiments, the results are combined in figure 2. For 3 weeks following the lacewing releases, aphid densities remained approximately constant in the release plots but expanded in the non-release, control plots. By the second week, the aphid population in the release plots was less than before release (ratio = 0.85 ± 0.16), while the aphid population had more than doubled in the control plots (ratio = 2.37 ± 0.67; t = 2.50, P = 0.03). This trend continued for a third week, but for the remaining period there was little or no difference between the two treatments (fig. 2). Thus, releases of extremely high densities of lacewing eggs produced only a modest and transient suppression of aphid populations. We suspect that releases conducted at the recommended rate would not have produced detectable results.
A few days after the lacewing eggs were released, few lacewing larvae could be found in any of the plots. This observation was consistent with many other observations where many adult lacewings and their eggs were seen, but few or no larvae were detected (fig. 1). However, a number of other generalist predators were present.
Predators attack lacewings
Direct observations in the field in 1991 and 1992 revealed that generalist predators, including nymphal big-eyed bugs and nymphal and adult assassin bugs, prey on lacewing larvae. Other predators that were abundant, for example damsel bugs, were never observed feeding in the field on any prey; thus, we were unable to determine if they preyed on lacewing larvae. To determine if generalist predators caused substantial mortality of lacewing larvae, and to quantify their effect on the biological control of the cotton aphid, we performed a manipulative experiment.
Methods
This experiment, conducted August 21–30, 1992, at the Kearney Agricultural Center, was designed to isolate the influences of each of three predators, big-eyed bugs, damsel bugs and assassin bugs, with and without the presence of lacewings, on lacewing survival and biological control of the cotton aphid. Individual plants harboring aphid populations were chosen for study. Each plant was carefully inspected to count all aphids and to remove all nymphal and adult predators. Polyester mesh sleeves were then placed over the plants to create a small cage (either the entire plant or the top portion of the plant was enclosed, depending on aphid density). Before sealing the sleeve cage, eight treatments, each replicated 6 to 10 times, were then applied to the caged plants: (1) no predators, an “aphids only” treatment; (2) aphids plus two big-eyed bug adults; (3) aphids plus two damsel bug adults; (4) aphids plus two assassin bug adults; (5) aphids plus five C. carnea lacewing larvae (second instar); (6) aphids plus five lacewing larvae plus two big-eyed bug adults; (7) aphids plus five lacewing larvae plus two damsel bug adults; (8) aphids plus five lacewing larvae plus two assassin bug adults. Lacewing larvae were obtained from a commercial insectary. After 7 to 8 days, the plant stems were cut and the sleeve cages brought into the laboratory, where all predators and aphids present were counted.
Fig. 1. Naturally occurring densities of lacewing eggs and larvae in six fields sampled during the late season (August 26 - September 2, 1992) in San Joaquin Valley cotton. Five plants were sampled in each field except for field S1, in which 48 plants were sampled. Field K had been treated with methamidophos June 24 for Lygus control, and field S3 had been treated with chlorpyrifos August 4 for aphid control; all other fields were never treated. Shown are means plus one standard error of the mean. No lacewing larvae were recovered from fields WS1, K, S2 or S3. WS = West Side Field Station; K = Kearney Agricultural Center; S = UC Cotton Research Station, Shafter.
Results
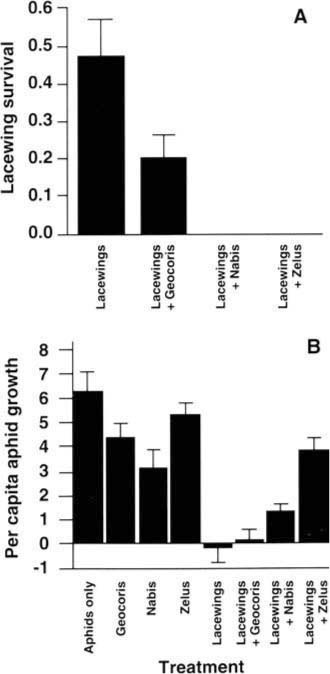
Survival of lacewing larvae varied strongly across the four treatments to which lacewings were added (fig. 3a). Survival decreased from 47% in the absence of predators to 20% when big-eyed bugs were present (P = 0.06), and to 0% when either damsel bugs (P = 0.001) or assassin bugs were present (P = 0.003). We can infer that the decreased lacewing survival was a result of predation rather than competition for aphid prey, because the availability of aphid prey was greater where predatory bugs were present. This negative effect of the generalist predators on lacewing survival influenced the level of biological control of the cotton aphid (fig. 3b). The first five treatments shown in figure 3b provide a test of whether each of the four predators considered alone has an impact on aphid population growth. When aphids were caged alone, their populations expanded rapidly, as expected. Assassin bugs and big-eyed bugs alone exerted minimal influences on the rate of aphid population growth (P > 0.10). Damsel bugs slowed aphid population growth substantially (P = 0.03), but did not control the populations. Lacewing larvae were, however, able to cause aphid populations to decrease rather than expand P = 0.001); the median response was a 91.2% decrease of aphid numbers.
This highly successful level of biological control produced by lacewing larvae feeding alone was disrupted when other predators were added. Although big-eyed bugs had a minimal effect (P = 0.15), the addition of damsel bugs allowed aphid populations to start growing again (P = 0.01), and the addition of assassin bugs appeared to remove most of the suppressive effect of the lacewings (P = 0.001; fig. 3b). Clearly, these predators did not act in an additive way to suppress aphid numbers. Rather, the heavy predation by damsel bugs and assassin bugs on lacewing larvae allowed aphids to escape the effective biological control produced by the lacewings alone. This result was surprising, especially for damsel bugs, which are known as major predators of aphids.
Fig. 2. Field experiment assessing the efficacy of augmentative releases of green lacewing eggs for biological control of the cotton aphid.
Conclusions
Biological control of the cotton aphid by generalist predators involves complex interactions among predators that have not previously been studied by biological control researchers. Densities of lacewing eggs are naturally high in San Joaquin Valley cotton fields; thus, augmentative releases of lacewings should only be contemplated after field scouting has shown that natural populations are not present in high densities. Our small plot releases suggest that releases of lacewing eggs at extremely high rates (much higher than is commercially viable given the cost of $3 to $4 per 1,000 eggs) produced only modest suppression of aphids. Recommended release rates would probably not produce detectable results.
Fig. 3. Influences of generalist predators on (A) lacewing larval survival and (B) percapita aphid population growth rates. Percapita aphid population growth was calculated as (final aphid numbers - initial aphid numbers)/(initial aphid numbers); a value of 0.0 indicates no change in aphid populations. Aphids were present in all treatments.
The key reason why naturally present populations and augmentatively released lacewing eggs do not control aphids appears to be the heavy mortality imposed on lacewing larvae by other generalist predators. Because our conclusions are based on only 2 years of study, additional work is required to determine how general our results are. We need to understand fully the interactions between lacewings and other generalist predators to predict late-season outbreaks of the cotton aphid.
Can we manipulate this system to increase the effectiveness of lacewings? We do not wish to suppress populations of big-eyed bugs or damsel bugs because these predators probably help control other cotton pests, including Lygus bugs, spider mites and worms. Thus, we may need to search for other biological control agents for the cotton aphid that are not susceptible to these predators, or develop other control tactics, such as host plant resistance or cultural techniques. Research continues on all of these fronts.