Calag Archive
Calag Archive
Temperature affects lesser mealworm populations in turkey brooder houses
Publication Information
California Agriculture 48(2):18-21.
Published March 01, 1994
PDF | Citation | Permissions
Abstract
The lesser mealworm beetle is a serious pest in turkey brooder houses. It tunnels into the building walls and insulation, and serves as a vector of poultry diseases and an intermediate host of parasites. This research showed that population growth is encouraged by certain temperatures and by certain industry practices, but more research is needed to evaluate the population dynamics of the lesser mealworm, particularly its response to varying temperatures.
Full text
The heat furnished by brooder stoves combined with the body heat from poults provide the optimum environmental temperature to maintain lesser mealworm populations.
In 1991, turkey ranked 17th among all California commodities, with a farm market value of $241 million. California was third in the nation in turkey production, with 29 million birds raised.
The lesser mealworm, Alphitobius diaperinus (Panzer), is an important insect pest associated with turkey production. It was first detected in California poultry houses approximately 35 years ago. The number of affected facilities and the severity of infestation has increased over the years. In California, the lesser mealworm is more abundant in turkey brooder houses than in turkey grow houses.
The lesser mealworm has become a major pest with the advent of large, modern brooding complexes in the turkey and broiler industries. Litter (wood shavings mixed with manure, spilled feed and water) on the floor of these houses has proven to be an ideal medium for the lesser mealworm's development. Economically, mealworms pose a threat because they may tunnel into the insulation of poultry buildings, thereby decreasing the effectiveness of the insulation and often necessitating its replacement.
In California, turkey growers also fear the lesser mealworm for its ability to be a vector of poultry diseases and parasites. An important pathogen of poultry transmitted by lesser mealworms is avian leukosis virus. The mealworm may also serve as an intermediate host for tapeworms, and it has been found to harbor Salmonella typhimurium and Escherichia coli. In addition, the mycotoxin F-2, produced by the fungus Fusarium roseum, may persist in the lesser mealworm through metamorphosis. The persistence of this mycotoxin through the beetle's various developmental stages raises questions about the ability of other pathogens to do the same thing. If transstadial passage is occurring, this could explain why certain diseases seem to be endemic to particular production units despite extensive cleaning and disinfecting efforts.
This study was designed to evaluate the population dynamics of the lesser mealworm in turkey brooder houses and to develop information on the response of these beetles to varying temperatures.
Life cycle of the lesser mealworm
The optimum temperature for the development of the lesser mealworm is approximately 90°F; at this temperature the mealworm develops from egg to adult in 45.6 days. No egg hatching occurs at temperatures above 100°F or below 70°F. Development stages include egg, larva (with as many as 11 instars), pupa, and adult beetle. The average time from egg to egg is about 71 days.
Strain differences. A strain of the lesser mealworm from a Southern California brooder facility was established in the laboratory at UC Riverside. All life stages of the California lesser mealworm were reared under various constant temperatures in the laboratory to determine respective development times and upper and lower development thresholds.
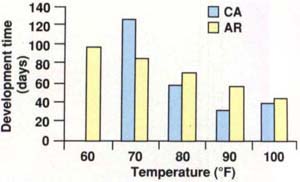
Temperature has a marked influence on the mealworm's life cycle. Figure 1 shows the development time of the California lesser mealworm compared to a strain of lesser mealworm from Arkansas. The Arkansas strain developed successfully from egg to adult at temperatures ranging from 60° to 100°F. Development times at these temperatures ranged from 97.2 days at 60°F to 42.3 days at 100°F. The California strain did not develop over such a wide range of temperatures; its development times ranged from 125 days at 70°F to 30.3 days at 90°F. Development slowed to 37.8 days at 100°F, indicating that this temperature was suboptimal; no similar relationship was demonstrated for the Arkansas strain. Differences in rearing conditions and feeding stress could explain some of the variation in development time between the two strains, but the California strain is clearly adapted to a much narrower range of temperatures than the Arkansas strain.
Chemical control of lesser mealworm. The increased incidence of lesser mealworm problems in turkey brooder houses has caused the California turkey industry to search for methods of control. Chemical control is currently the only cost-effective method for suppressing the population. However, recent population-monitoring studies in California have determined that chemical applications approved for use in poultry houses do not provide effective control.
A limited research base is available from which to develop an integrated control program for lesser mealworms in poultry brooder houses. Insecticides registered for use include carbaryl, malathion, permethrin, Ravap (tetrachlorvinphos+dichlorvos) and boric acid. No biological or cultural controls have been defined.
Brooder house conditions
The study was carried out in uninsulated brooder houses 40 feet wide by 416 feet long. To provide supplemental heat, each house contained two rows of 26 brooder stoves, one row on each side of the house. The stoves were placed approximately 6 feet from the outside wall and 8 feet from the center of the house.
Litter management prior to trial. After each brood, each house was hosed down with water at 250 psi. Then the used litter was scraped out one end of the house with a tractor and scraper. After washing and litter removal were completed, the house was disinfected with a quaternary ammonia product using the same highpressure system used for washing. An insecticide was sprayed on the walls and ceiling. Also, one of two boronbased insecticides was placed on the floor of the house at a rate of 2 lb per 100 ft2. Approximately 4 inches of clean litter was distributed over the boron powder.
Temperature monitoring. Temperatures were measured with 24 thermocouples placed approximately 2 inches into the soil (ground) and another 24 thermocouples placed between the soil and the litter (interface). Thermocouples were also placed 12 ft above the floor one quarter of the distance from both ends of the house, and one each at 4 and 8 ft above the floor in the center of the house (ambient). A Campbell Scientific CR-5 digital recorder recorded temperatures hourly.
Census method. Adult and larval stages of the lesser mealworm were monitored weekly in the test houses at multiple fixed points for 2 years and 4 months. The monitoring tool was an Arends tube trap, a rolled piece of cardboard (8 by 12 inches) inserted into a piece of rigid polyvinyl chloride (PVC) pipe (9.2 inches long by 1.52 inches in diameter).
In addition, every week the relative population density of the mealworms was checked visually at the same locations. This was done by digging into the litter around the perimeters of the first two stoves on the west side and the first stove on the east side of the south end of the house. Then the number and instar stage of the mealworms found in the traps and in the litter at these locations were compared.
Mealworm population growth
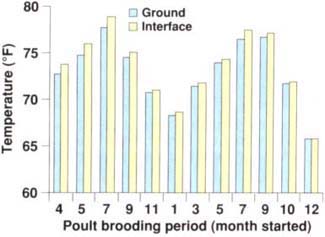
The original reason for placing thermocouples 2 inches into the soil of the floor of the brooder house was to establish temperature differences between the interface (where litter meets soil) and the soil. The soil temperature was found to be essentially the same as the temperature at the interface (fig. 2). With this established, we decided to look for relationships between mealworm numbers and temperatures of the environment (outside), the in side of the house (ambient) or the interface.
Fig. 1. The development time for the Arkansas strain of lesser mealworm ranged from 97.2 days at 60°F to 42.3 days at 100°F. The California strain ranged from 125 days at 70°F to 30.3 days at 90°F, with development slowing to 37.8 days at 100°F.
Fig. 2. The ground temperature (2 inches into the soil) varied by less than 1°F from the interface temperature (between the soil and litter).
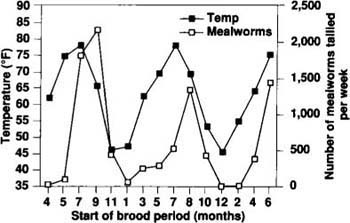
Fig. 3. The number of mealworms tallied per week increased with rising outside temperatures, lagging the rise by a week.
In the trials, two columns of brooder stoves were placed 6 feet from the outside wall and 8 feet from the center of the house. In this view, stoves have been raised to the ceiling. PVC pipes on the center posts hold temperature sensors.
Correlation of population with temperature. As figure 3 shows, the mealworm population in turkey brooder houses responds to seasonal fluctuations in temperature and follows the rise and fall of the temperature outside the house. The brooder stoves, used for 4 weeks out of the 6-week brood cycle, combined with the body heat from poults, keep the temperature high enough to maintain the mealworm population when outside temperatures are below their development threshold. The increase in the outside temperature during spring and summer is followed by a corresponding increase in the number of mealworms in the houses.
Correlation of population with chemical control. Our monitoring of mealworm populations coincided with the first use of a relatively new boronbased insecticide. This new product has a labeled use rate of 2 lb/100 ft2 of floor area at the onset of the brood, with an additional pound applied to the same area 9 months later. Technical personnel representing the manufacturer of this product stated that their control recommendations were based on the production of broilers on the East Coast, where broiler litter is replaced yearly and therefore very little of the insecticide is lost between brood changes.
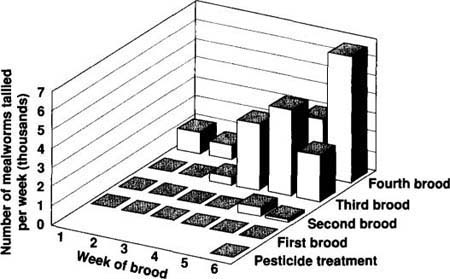
The boron-based insecticide was applied on the floor with the intention of killing adult mealworms at the interface and also larvae burrowing into the soil to pupate. New adults emerging from pupal burrows would come in contact with the material. Prior to the introduction of the new insecticide, we would have expected an increase in mealworm numbers by April. Following the introduction of the insecticide, we found almost no mealworms for this first brood. Small numbers began to appear in the fifth and sixth weeks of the second brood. By the third brood, numbers were back to pretreatment levels. (Figure 4 shows the progression.) We conclude that the practice of scraping the litter out after each turkey brood may have removed most of the insecticide after the initial brood.
Integrated control needed
To allow integrated control technologies to be developed, much more information is needed on the behavior and biology of the lesser mealworm in poultry houses. This study is a beginning.
Role of brooder stoves in population dynamics. During the course of this study, interface temperature was less than 80°F in the brooder house, not high enough to account for the observed increase in mealworm numbers. As was seen with mealworms maintained at constant temperatures, rapid development occurs at temperatures near 90°F. The use of brooder stoves to provide heat to turkey poults for the first 4 weeks of each new brood provides the supplemental heat necessary to account for accelerated mealworm development.
Chemical control. Adult and larval mealworm numbers can be greatly reduced, but not eliminated entirely, with an integrated control program combining cultural and chemical practices such as using insecticides during cleanup. However, larvae that have burrowed into the dirt floor of the house in search of a pupation site are not controlled by these insecticide applications.
Fig. 4. The number of lesser mealworms expected during the spring was suppressed by a boron-based pesticide applied to the soil. By the third brood after the treatment, however, the number was at the expected level.
Litter management. When boron products are applied, the practice of scraping out the litter after each brood apparently allows many adult mealworms to emerge unharmed from the soil, because the boron compounds they would otherwise have contacted have gone out with the litter. The result is a rapid population increase during subsequent broods.
Reinfestation. Complete elimination of the lesser mealworm from infested premises is difficult with some current husbandry practices. Reinfestation has been observed to occur in several ways. The practice of scraping used litter from brooder houses into a pile just outside the end door encourages reinfestation. Frequently, after a house is cleaned and disinfected, new litter is delivered to the opposite end of the house and scraped into the house before the pile of used litter is removed. Adult lesser mealworms have been observed moving at night from the used litter pile to other locations, including the cleaned and disinfected house.
Mealworm larvae may leave the house and burrow into the soil outside the house to pupate. The emerging adults may return to the house. Migrating larval stages have been found in the walls and the insulation, and pupating has been observed in the dust between the walls and in the insulation.
Probably the most insidious source of reinfestation is pupation in the floor of the house, untouched by the removal of the used litter and disinfection of the house before the next brood arrives. By the time the new brood is in place, newly emerged adult mealworms will be in the clean house and potentially carrying disease organisms from the previous brood.