Calag Archive
Calag Archive
A better tick-control trap: Modified bait tube controls disease-carrying ticks and fleas
Publication Information
California Agriculture 52(2):43-47. https://doi.org/10.3733/ca.v052n02p43
Published March 01, 1998
PDF | Citation | Permissions
Abstract
In northwestern California, the dusky-footed wood rat is a primary reservoir of Lyme disease (LD) spirochetes and an important host of three tick species that collectively transmit the causative agents of LD, human granulocytic ehrlichiosis, Rocky Mountain spotted fever and tularemia. It also is infested by two flea species that can transmit the agent of plague. We refined a method of applying pesticides to the host using liquid permethrin-treated bait tubes, and found it to be highly effective for reducing populations of these ticks on wood rats in brushlands. Although permethrin products are not currently registered for this use, this approach offers a promising tool for controlling arthropod-borne diseases in the western United States.
Full text
Ticks represent the most important group of arthropods among those that transmit microbial disease agents to humans in California and in the United States at large. Since the mid-1970s, several newly emerging tick-borne infections have been recognized in this country, most notably Lyme disease (LD), human babesiosis, human granulocytic ehrlichiosis and human monocytic ehrlichiosis. Human infections with these microbial agents can range from inapparent to moderate or severe; in the case of babesiosis and ehrlichiosis, they are sometimes fatal. Of these, LD alone accounts for more than 90% of the total number of reported cases of arthropod-borne human diseases. In 1996, for example, 16,461 cases of LD were reported to the Centers for Disease Control and Prevention (CDC) by 45 states and the District of Columbia (MMWR 1997). This represents a precipitous rise since 1982 when 491 cases were reported nationwide.
Because of the growing recognition of the importance of emerging tick-borne infections, there has been a recent upsurge of interest in personal protection methods (Lane 1989) and in developing novel approaches for reducing natural tick populations. Tick populations can be managed by habitat modification directed at either plant or vertebrate hosts, by reproductive interference, by the use of parasitoids and predators, and by integrated pest management strategies (Schmidtmann 1994). Anti-tick measures that use host animals to carry pesticides targeting ticks, such as host-targeted methods, can be effective and economical, and they reduce treatment volumes during pesticidal applications while minimizing nontarget effects (Schmidtmann 1994).
Left to right: Female, male, nymph and larval forms of the western black-legged tick. This tick is the primary vector of the Lyme disease spirochete in California.
Lyme disease basics
Lyme disease, or Lyme borreliosis, is caused by a spirochetal bacterium that is transmitted to humans and other animals by infected ticks. For infection to occur, an infected tick must remain embedded in the skin for at least 24 hours to several days, depending on the tick species involved and its life stage. Experimental evidence suggests that adult ticks may take longer to transmit the infection than nymphal ticks.
Signs and symptoms of infection appear 3 to 32 days after an infected tick attaches. They frequently include a slowly expanding rash around the tick bite, (ever,' aches and fatigue. The reddish lesion may be absent in 20% to 40% of people with early-stage infection. Lyme disease may also be diagnosed through a two-tiered blood test.
The sooner Lyme disease is treated, the more likely it will be cured. Doctors advise immediate treatment with antibiotics; without treatment, people may suffer arthritis, heart block or nerve damage months or even years later.
In California, the western black-legged tick is primarily responsible for spreading the disease to humans. Lyme disease spirochetes typically occur in about 1% to 3% of the adult and nymphal western black-legged ticks, but the rates can be higher in some areas. Infection of nymphs can be 3 to 4 times higher than in adult ticks. Nymphs are also easy to overlook because they are no bigger than a poppy seed when they attach. The highest reported nymph infection rate has been in Mendocino County where 13.6% of nymphal ticks at one site in Potter Valley contained spirochetes in 1995. (Related story, p. 4)
One innovative host-targeted approach, the insecticide-bait-box method, has been modified several times since its development for plague control during the late 1950s (e.g., Kartman 1958; Sonenshine and Haines 1985; Gage et al. 1997). Wild rodents are attracted to a bait housed within a container open at one or both ends. Upon entry, their fur becomes coated with a pesticide lethal for ectoparasites, that is those parasites that live on the exterior of their hosts. In Colorado, this approach was adapted for controlling fleas and ticks on the Mexican wood rat (Neotoma mexicana) in foothills along the Rocky Mountain Front Range (Gage et al. 1997). This wood rat and one of its ticks, Ixodes spinipalpis, were found to maintain the LD spirochete (Borrelia burgdorferi) in a transmission cycle that does not involve humans. The tick Ixodes spinipalpis feeds upon various rodents, rabbits and hares, but it rarely bites humans.
In Northern California, the dusky-footed wood rat (Neotoma fuscipes) and the tick I. spinipalpis maintain a similar transmission cycle of the LD spiro-chete B. burgdorferi and related spiro-chetes (Brown and Lane 1992). The wood rat also is fed upon by the western black-legged tick (Ixodes pacificus), which serves as a bridge vector of B. burgdorferi to humans and has been implicated as a vector of the rickettsia that causes human granulocytic ehrlichiosis (Barlough et al. 1997). Other ectoparasites that feed on wood rats in this region include the Pacific Coast tick (Dermacentor occidentalis), which is infected naturally with the agents of Rocky Mountain spotted fever and tularemia, and the fleas Orchopeas sexdentatus and Malaraeus telchinus, which can transmit the bacterium causing plague.
Because of the importance of the dusky-footed wood rat as a host of various microbial agents and their arthropod vectors, the present study was undertaken to determine the efficacy of the CDC version of the bait tube method (Gage et al. 1997) for controlling its associated ectoparasites. To this end, we selected a field site in northwestern California that has an abundant population of wood rats with a diverse assemblage of ectoparasites. Although the pesticide we tested is not currently available for commercial use, our study shows the experimental method is highly effective for reducing populations of vector ticks and fleas in rural settings.
Rodent, ectoparasite collections
The study was performed at the UC Hopland Research and Extension Center (HREC) in southeastern Mendocino County. The HREC is a multipurpose agricultural sciences research facility located approximately 100 miles north of the University of California at Berkeley. It comprises 5,358 acres of hills interspersed with ravines on the western slopes of the Mayacmas Mountains. The climate is Mediterranean with cool moist winters and hot dry summers.
Rodents were usually trapped 1 to 2 nights per month per site from April to September 1995 and from March to June 1996 at each of two chaparral (brushland) sites spaced ∼0.3 miles apart. Preliminary trapping was conducted in April 1995 to identify 40 active wood rat nests at both sites, and systematic trapping began in May 1995. To capture wood rats, 1 Tomahawk trap (Tomahawk Live Trap, Tomahawk, WI) was placed adjacent to each active nest. To collect other small mammals, 1 Sherman live trap (H.B. Sherman Traps, Tallahassee, FL) was set within 5.5 to 11 yards of each nest. All traps were baited with a mixture of molasses, corn, oats and barley (Valley View Feed, San Pablo, CA).
Captured mammals were placed individually inside a plastic bag and anesthetized with inhalant methoxy-flurane (Metofane, Pitman-Moore, Mundelein, IL). They were then identified by species, age and sex; weighed; examined for the presence of ectoparasites, and ear-tagged (National Band & Tag, Newport, KY). The entire body of each mammal was inspected for ectoparasites for 3 to 5 minutes, and fleas were removed with combs and ticks with tweezers. All ectoparasites were preserved in 70% alcohol for later identification with published keys. Animals were returned to their traps and, following recovery from anesthesia, released at their sites of capture.
Permethrin-treated bait tubes
The bait tubes consisted of polyvinyl chloride cylinders 4 inches in diameter and 20 inches long. Each tube was lined internally at both ends with a narrow ring of carpet (0.6 inch thick by 2 inches wide). The carpet was fastened to the tube at one end with a water-resistant adhesive (Lokweld 600, Ralph Wilson Plastics Co., Temple, TX). To facilitate insertion of the bait block, the carpet strip at the opposite end was made removable by attaching separate pieces of Velcro to the nonfabric side of the carpet and to the inner surface of the bait tube with another adhesive (Weldbond, Frank T. Ross & Sons, Richmond, IL).
To attract rodents, a cylindrical block of bait reinforced internally with two wire-mesh cylinders having mesh diameters of 0.47 and 0.12 inches was fastened in the center of each tube with wire. The bait consisted of cracked corn embedded in paraffin at a ratio of 4 to 1 by volume. Bait blocks were replaced monthly after each rodent trapping session. One bait tube was placed next to each active wood rat nest in the treated (n = 40) and untreated areas (n = 40). To secure bait tubes in the environment, several stones were put alongside and on top of each tube. The tubes were set out initially on May 19, 1995, removed Aug. 18, 1995, and set out again on April 19, 1996, until late June 1996 when the study ended.
In the treatment area only, the carpet rings at both ends of the bait tubes were treated with about 0.34 fluid ounces (10 ml) of Permanone Pyrenone Liquid Dust (Fairfield American Corporation, Rutherford, NJ), which contains 1.250% permethrin, 0.125% pyrethrins, 10.000% piperonyl butoxide and 88.625% inert ingredients. Permethrin is of low mammalian toxicity and is not known to be harmful to wood rats or other wild rodents. The pesticide was applied five times (on May 19, June 20 and July 21, 1995, and on April 19 and May 24, 1996) and rodent trapping was performed 26 to 32 days later, except after the July 21, 1995, application. After July 21, 1995, trapping was conducted on four occasions before pesticide was reapplied, after 25, 60, 248 and 270 days. This was done to determine how long the pesticide was able to effectively suppress populations of ectoparasites.
Statistical analyses
The chi-square test with Yates's correction was used to test for differences in the prevalence of ticks or fleas on wood rats captured in the treated versus the untreated area on all sampling occasions. Fisher's exact test (two-tailed) was used to compare the prevalence of these ectoparasites on brush mice (Peromyscus boylii) from both areas on three occasions. For purposes of this analysis, all tick species were pooled because of their combined low abundance on most collection dates. Insufficient numbers of the other mammalian species caught precluded statistical comparison of their ectoparasite loads on all dates. A 5% level of significance was chosen for rejection of the null hypothesis.
Small mammal captures
Trapping resulted in 562 wood-rat captures on 29 dates between April 26, 1995, and June 27, 1996; 191 different individuals were captured. Comparable numbers of wood rats were collected in the untreated (n = 273) and treated areas (n = 289). Overall, 67% and 65% of the wood rats caught in the untreated and treated areas, respectively, were recaptured animals. We collected five additional species of small mammals including 105 brush mice, 24 California kangaroo rats (Dipodomys californicus), 22 deer mice (Peromyscus maniculatus), 20 pi$nTon mice (Peromyscus truei), 9 Sonoma chipmunks (Tamias sonomae), 1 undetermined shrew (Sorex sp.), and 1 brush rabbit (Sylvilagus bachmani).
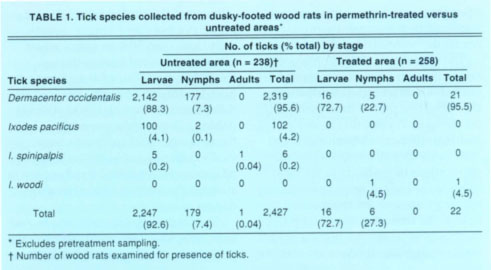
TABLE 1 Tick species collected from dusky-footed wood rats in permethrin-treated versus untreated areas*
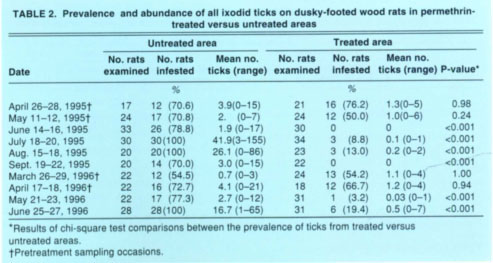
TABLE 2 Prevalence and abundance of all ixodid ticks on dusky-footed wood rats in permethrin-treated versus untreated areas
Efficacy of treatment
Exclusive of pretreatment sampling in 1995 and 1996, 2,427 ticks of 3 species were removed from wood rats in the untreated area, which is 110 x the number of ticks of 2 species found on this host in the treated area (table 1). In both areas, the Pacific Coast tick was by far the most abundant tick. Low numbers of the western black-legged tick and the tick I. spinipalpis occurred on wood rats in the untreated area, and one I. woodi tick infested a wood rat in the treated area. Besides ticks, wood rats yielded 342 fleas in the untreated area and 21 fleas in the treated area, a 16-fold difference (data not shown). Orchopeas sexdentatus comprised 99.1% of all fleas identified from wood rats during pretreatment and post-treatment sampling; the remainder were Malaraeus telchinus.
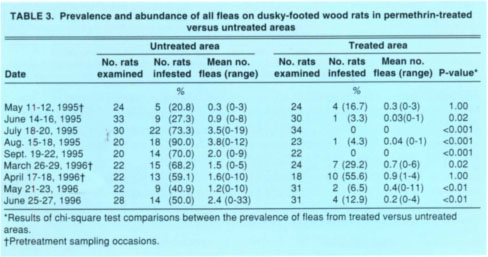
The prevalence of ticks on wood rats in the untreated versus treated areas did not differ significantly during the initial two pretreatment sampling occasions each year, that is, in April and May 1995 or in March and April 1996 (table 2). Following individual applications of pesticide to the bait tubes in the treated area, however, significantly more wood rats yielded ticks in the untreated area for up to 2 months post-treatment. Similar results were obtained for fleas except that the efficacy of the pesticidal treatment may have persisted longer. Specifically, less than 50% as many wood rats in the treated versus the untreated area produced fleas 8 months post-treatment in March 1996, and this difference was statistically significant (table 3). Because the prevalences in the two areas post-treatment were vastly different and statistically significant, we considered it redundant to statistically compare the mean abundances as well.
On three sampling occasions in August 1995, May 1996 and June 1996, the prevalence of ticks on brush mice from the treated versus the untreated area differed significantly only once. However, few brush mice were captured during each of these collection period (7 to 13 mice in the untreated area versus 8 to 11 mice in the treated area) and their tick loads averaged just 0 to 1.1 per animal per site. The abundance of fleas was even lower (0 to 0.4 per animal per site), and the prevalence of fleas on brush mice was similar in both areas.
TABLE 3 Prevalence and abundance of all fleas on dusky-footed wood rats in permethrin-treated versus untreated areas
Control of vector-borne diseases
As shown recently in Colorado (Gage et al. 1997), the liquid permethrin-treated bait tube method reduces populations of ticks and fleas on wood rats. Our research demonstrates that permethrin effectively controls ticks for at least 2 months and fleas for up to 8 months. In Colorado, fleas were reduced for more than 7 months after treatment. We do not know why treatment controls ectoparasites for so long, but permethrin residues persist well and wood rats may shed pesticide-coated hairs in their nests (Gage et al. 1997).
Lower numbers of ectoparasites on Mexican or dusky-footed wood rats following treatment could be due to the direct toxicity of permethrin, its repellency, or both. In the treated area, we never observed dead ticks attached to wood rats. This suggests that host-seeking ticks were incapacitated, killed or repelled soon after contact with treated animals. We speculate that permethrin killed such ticks, but further studies on its repellency are warranted. Most Pacific Coast ticks and soft ticks (Ornithodoros coriaceus) exposed to permethrin-treated cotton surfaces were repelled initially for up to 8–15 min. (Lane and Anderson 1984).
The current study was performed at the identical field-sites where another host-targeted method for delivering permethrin to wood rats was tested in 1992 and 1993 (Leprince and Lane 1996). In that study, permethrin-impregnated or untreated cotton balls were dispensed in galvanized metal cylinders, as potential nesting material, next to 95 wood rat nests. This method was ineffective for controlling ticks associated with wood rats, but it appeared to be useful for reducing populations of the flea O. sexdentatus. The failure to achieve tick control was related directly to the reluctance of wood rats to incorporate cotton balls into their nest cups. Only 2 of 8 active wood rat nests examined had small quantities of cotton fibers interwoven into the nest cups.
The lack of significant differences among the prevalences of ticks or fleas on brush mice from both sites on most sampling occasions may indicate that this rodent is not attracted to the bait stations. Alternatively, if brush mice are attracted, the entryway diameter of the bait stations, which were designed specifically for the much larger sized wood rat, may be too large to efficiently treat small mice as they enter the tubes. It is equally conceivable that the low sample sizes of mice and their low ectoparasite burdens concealed any differences that may have existed between sites. With respect to their diets, the bait used in the present study (cracked corn) should have been as attractive to brush mice as it was to wood rats because the herbivorous diets of these rodents overlap (Jameson, Jr. and Peeters 1988).
Several modifications of the original design (Gage et al. 1997) were necessary to adapt the bait tube method to conditions in a chaparral plant community. One major obstacle encountered was the rapid disappearance of bait, usually within several days of placement, because of the high diversity and abundance of rodents in brushland. A second obstacle was that of locating adhesives that would effectively bind the carpet and the Velcro to the bait tubes. We solved both problems, which facilitated the research and increased the cost-effectiveness of the method by reducing the amount of bait, the time required to prepare it, and the frequency of field trips to replenish it. Reinforcing the internal portion of each bait block with wire-mesh cylinders prevented rodents from ingesting all the bait while continuing to attract them for 1 month or more without replenishment. Moreover, after a process of trial and error, two adhesives were found that fastened the carpet and Velcro to the tubes more securely and longer than any of the other adhesives evaluated (n = 5).
We conclude that the modified liquid permethrin bait tube method is highly effective in reducing populations of vector ticks and fleas on wood rats in brushlands. Since 3 of the 4 tick species infesting wood rats can transmit one or more bacterial agents, and the fleas O. sexdentatus and M. telchinus are capable of transmitting the plague bacterium, this approach offers considerable promise as a tool for reducing arthropod vectors of human pathogens in rural and possibly peridomestic settings in the western United States. This point is underscored by the fact that the method has proven effective against ticks and fleas in dissimilar environments in Colorado and California. Moreover, this host-targeted method is cost-effective, timesaving, and employs a pyrethroid pesticide that is persistent, effective at low doses and of low mammalian toxicity (Gage et al. 1997).