Calag Archive
Calag Archive
Insecticides sought to control adult glassy-winged sharpshooter
Publication Information
California Agriculture 55(4):22-27. https://doi.org/10.3733/ca.v055n04p22
Published July 01, 2001
PDF | Citation | Permissions
Abstract
The bacterium that causes Pierce's disease (Xylella fastidiosa) is transmitted to grapevines by the glassy-winged sharpshooter (GWSS). Insecticides were evaluated for efficacy and residual activity against adult GWSS on grapevines. Ten insecticides were tested in the cyclo-chlorinated, carbamate, organic phosphate, pyrethroid and neonicotinoid chemical classes. Results from field trials indicate that the pyrethroids and neonicotinoids are promising control agents. Information on efficacious and environmentally compatible chemical control will be helpful in developing integrated pest management to protect California vineyards from Pierce's disease, as well as insecticide resistance management within crop-management production systems.
Full text
Above, An adult glassy-winged sharpshooter feeds on a grape cane. Right, The center leaves of grapes display classic Pierce's disease symptoms and hard "raisined" grapes beneath.
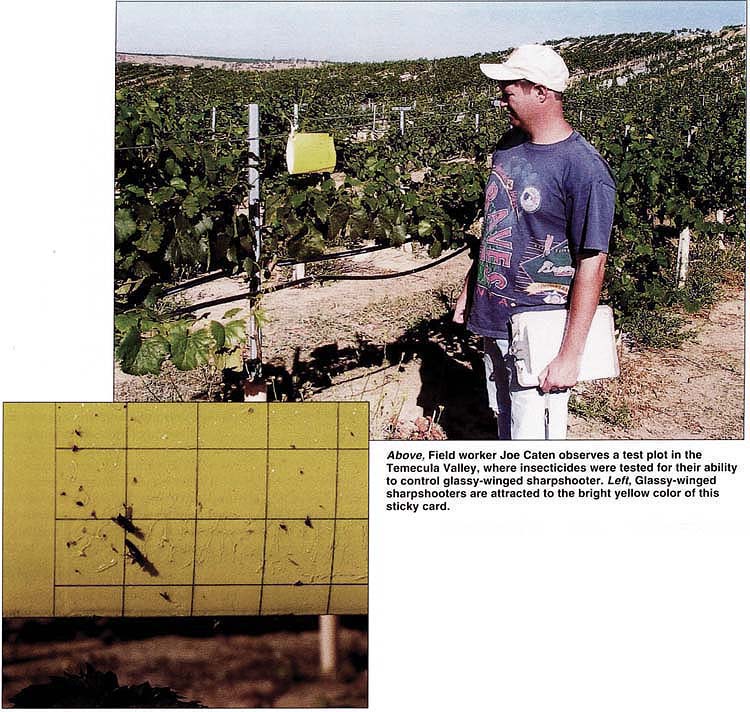
Above, Field worker Joe Caten observes a test plot in the Temecula Valley, where insecticides were tested for their ability to control glassy-winged sharpshooter. Left, Glassy-winged sharpshooters are attracted to the bright yellow color of this sticky card.
The glassy-winged sharpshooter (GWSS) is a primary vector of the bacterium (Xylella fastidiosa) that causes Pierce's disease in grapevines. Since GWSS populations were discovered in Southern California, Pierce's disease has increased greatly and resulted in serious fruit and vine losses. GWSS, Homalodisca coagulata, were likely introduced in California before 1990 on nursery stock from southeastern United States (Blua et al. 1999). They were first reported in Orange and Ventura counties in 1990 by Sorenson and Gill (1996). With the continuing spread of GWSS, Purcell and Saunders (1999) suggested that diseases caused by X. fastidiosa, such as almond leaf scorch and alfalfa dwarf disease (Davis et al. 1980; Hewitt et al. 1949), are likely to become more prevalent.
Our studies were prompted by the need for immediate action to safeguard Southern California vineyards and provide stopgap protection methods for crops and ornamentals across the state from Pierce's disease. An ecologically acceptable but efficacious chemical control program could be an essential component of integrated pest management (IPM) strategies to reduce GWSS populations. This would require knowledge of insecticidal chemistries effective against GWSS. Also, prudent use of insecticides requires that IPM, insecticide resistance management (IRM) and integrated crop management (ICM) programs be devised and implemented.
This research identifies groups of insecticides for use against GWSS and provides information for selectively choosing conventional insecticides as components of management programs.
Four insecticide trials
We conducted four experiments from July to early December 2000, in 2.9 acres of ‘2131 Chardonnay’ grapevines in Temecula, Calif. (Riverside County). We constructed two randomized, complete block designs with rows spaced on 10-foot centers and 6-foot vine spacing. In the first design, the center area of the 2.9 acres was used. Eighteen plots were divided into three replicates of six (plots) treatments. Each plot was 0.11 acres in size (experiments 1, 2 and 4). In the second design, the vineyard area on each side of the center area was divided in two halves. Each block half was divided into 12 plots, and the resulting 24 plots were arranged in three replicates with equal numbers of plots in each replicate of eight treatments (experiment 3).
Above, Trials were conducted on plots of '2131 Chardonnay'. Left, Cages containing 25 glassy=winged sharpshooters were enclosed with netting around grapevines.
GWSS adults were collected from UC Riverside citrus orchards and transported to the Temecuia vineyard. Cages were made by enclosing one vine in a plot with netting. GWSS were placed in cages 24 hours before insecticide applications or exposure periods. In pretrial tests, GWSS survival in cages sprayed with water was compared to survival in unsprayed cages. We also determined spray quantities that penetrated and reached the vines in the caged versus the uncaged vines. Plant Sciences, Inc. personnel made the pretrial and insecticide applications with a windmill air-blast sprayer (table 1).
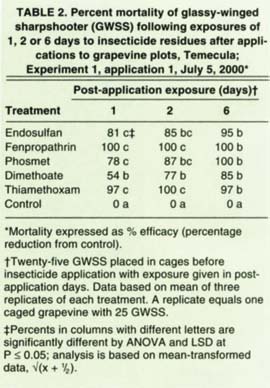
TABLE 1. Names, chemistry class, formulation and rate per acre of Insecticides evaluated for adult glassy-wing sharpshooter control, Temecula
TABLE 2. Percent mortality of glassy-winged sharpshooter (GWSS) following exposures of 1, 2 or 6 days to insecticide residues after applications to grapevine plots, Temecula; Experiment 1, application 1, July 5, 2000*
For experiment 1, the first of two applications were made the first week of July, and the second a week later. Treatments were (1) endosulfan (Thiodan); (2) fenpropathrin (Danitol); (3) phosmet (Imidan), applied for the first application followed by methomyl (Lannate) for the second; (4) dimethoate; (5) thiamethoxam (Actara); and (6) an unsprayed control. Except for treatment 3, the same chemicals were used in both applications.
GWSS were counted 6 hours after the first applications and then on 1, 2 and 6 days post-application. Then GWSS were removed from the cages and new populations were introduced. The second applications were applied 7 days after the first. GWSS were counted on days 1, 2 and 3 after the second applications. Then on post-application day 7, all GWSS were removed from the cages and a new population was again put in each cage. GWSS were counted after 24-and 48-hour exposures. This routine was continued at about weekly intervals for 29 days after the second application.
Treatments for experiment 2, starting in August, were the same as for experiment 1 except that a single-application treatment of bifenthrin (Capture) replaced the phosmet followed by methomyl treatment. GWSS in the cages were counted, removed and replaced weekly for 15 days as previously described for activities following the second applications of experiment 1.
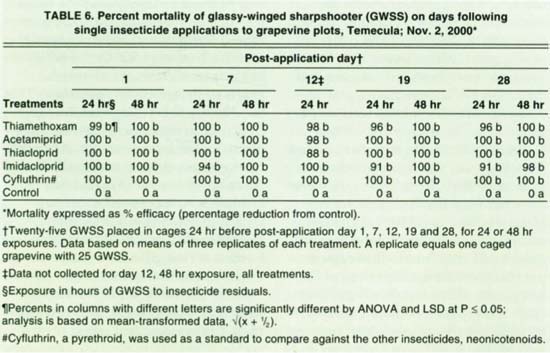
Experiments 3 and 4 were conducted similarly. Post-treatment counts and GWSS reintroductions into cages were continued for 28 days. Experiment 3 treatments, starting in September, were bifenthrin and an untreated control. For experiment 4, starting in November, treatments were the neonicotinoids — thiamethoxam, acetamiprid (Assail), thiacloprid (Calypso) and imidacloprid (Provado); a pyrethroid, cyfluthrin (Baythroid); and an untreated control.
Effectiveness compared
Pretrial test results.
At 24 hours after water spray, there was no significant difference in the numbers of GWSS alive in sprayed versus unsprayed cages. Spray pattern analysis showed that coverage in caged vines was 92% of the coverage in uncovered vines. Spray penetration tests were repeated twice with similar results during the season as canopy density increased. Spray pattern data analyses showed good coverage.
Experiment 1.
Fenpropathrin and thiamethoxam were the fastest-acting chemicals with 97% to 100% mortality of GWSS occurring within 24 hours of application (table 2). In fenpropathrin-treated plots, mortality was 100% in 6 hours. Dimethoate efficacy at 24 hours after the first application was inferior to other insecticides tested. GWSS mortality increased with time of exposure to endosulfan, phosmet and dimethoate residuals. Following the second application, 100% mortality of GWSS occurred for fenpropathrin after 24-or 48-hour exposures to 1-and 8-day residues. This compared to thiamethoxam, which caused the same mortality after exposure to 1-day residues, but 88% and 99% mortalities after 24-and 48-hour exposure to 8-day residues (table 3). Endosulfan, phosmet followed by methomyl, and dimethoate were ineffective except after high mortalities for 24-and 48-hour exposures to 1-day-old residues.
Experiment 2.
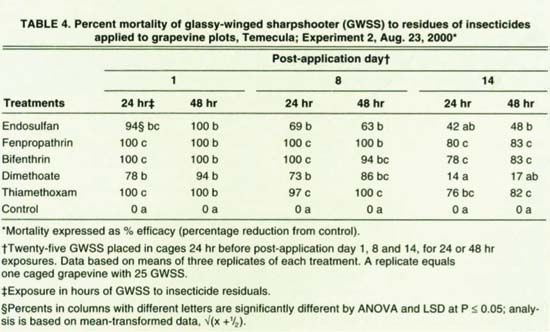
Fenpropathrin, bifenthrin and thiamethoxam were the most effective insecticides with 94% to 100% mortalities on day 8 after application (table 4). One and 2 days after application, GWSS mortalities on 1-to-2-day-old endosulfan residues were 94% and 100% compared to lower mortalities on residues on days 8 and 9, and days 14 and 15 after application. Dimethoate was the least effective treatment, with 73% mortality after a 24-hour exposure on day 8 following application.
Experiments 3 and 4.
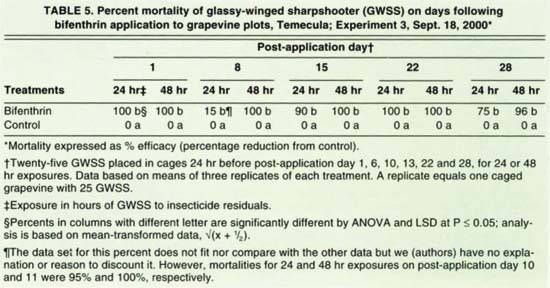
Bifenthrin efficacy for 48-hour exposures to 23-day-old residues was 100%, and 96% for 24-hour exposures to 28-day-old residues (table 5). Mortality after 24-hour exposures on day 6 after application was only 15% and remains unexplained. For experiment 4, the neonicotinoids (thiamethoxam, acetamiprid, thiacloprid and imidacloprid) and the pyrethroid (cyfluthrin) resulted in 91% to 100% GWSS mortalities over the 28-day duration of the experiment (table 6). About three-quarters of the vine foliage was desiccated at the start of the experiment and defoliation was mostly complete by the end of the test.
TABLE 3. Percent mortality of glassy-winged sharpshooter (GWSS) to residues of insecticides on days following second applications to grapevine plots, Temecula; Experiment 1, application 2, July 12, 2000*
TABLE 4. Percent mortality of glassy-winged sharpshooter (GWSS) to residues of insecticides applied to grapevine plots, Temecula; Experiment 2, Aug. 23, 2000*
Classes of insecticides evaluated to control glassy-winged sharpshooter included cyclo-CI, carbamate, pyrethroid and neonicotinoid. Chris McElliot collected glassy-winged sharpshooter from citrus for the trials.
Promising insecticides
The pyrethroids (bifenthrin, fenpropathrin and cyfluthrin) and the neonicotinoids (thiamethoxam, acetamiprid, thiacloprid and imidacloprid) appear to be promising for GWSS control. Of these, fenpropathrin, imidacloprid and cyfluthrin are in the current “Guidelines for GWSS management” (UC/USDA 2001).
TABLE 5. Percent mortality of glassy-winged sharpshooter (GWSS) on days following bifenthrin application to grapevine plots, Temecula; Experiment 3, Sept. 18, 2000*
Our experience in pest-insect crop protection has demonstrated that different insecticides within the same chemical class often have different qualities that contribute to their usefulness, when applied alone or in tank mixes with another insecticide in a different or even the same class. To address the total pest and beneficial arthropod populations present in vineyards and associated ecosystems, selection of the best-fitting insecticides becomes a critical imperative to implement IPM, IRM and ICM programs. Also, insecticide options must accommodate management alternatives for different growers and in different areas varying in climate, pest complex, and environmental and economic parameters.
In future studies, the efficacy of insecticides should be evaluated relative to potential interference with GWSS as a vector of X. fastidiosa. For example, soil-applied imidacloprid has been observed to suppress feeding activity of sucking insects (personal communication, M. Blua, UC Riverside) and may have potential in this respect. Likewise, in this study, given that fenpropathrin killed all the exposed GWSS present within 6 hours, it will be important to conduct studies to determine if this rapid insecticidal action could play a role in significantly reducing X. fastidiosa transmission to hosts by GWSS. Supporting studies are also needed to verify this quick insecticidal action by fenpropathrin and determine if other pyrethroids act similarly.
Sprays were applied with a tractor and air-blast sprayer. Several pyrethroid and neonicotinoid insecticides showed promising results.
Seasonal changes (shorter day lengths and lower temperatures) occurred during our experimental period from July to December 2000. Shorter insecticidal residual effectiveness was observed in midsummer experiment 1 compared to increasingly longer residue effectiveness in the early fall experiment 3 and the late fall experiment 4. Temperature, ultraviolet light and microbes may have caused more insecticidal degradation during the summer compared with fall conditions. Rain was not a factor in any of the experiments. GWSS were older in the fall than they were in the summer, and older insects may be more susceptible to insecticides. Future studies should elucidate interactions among seasonal variables and insecticidal degradation parameters with GWSS age and insecticide susceptibility. Because this work was conducted in two seasons, conclusions should be drawn within experiments and caution must be used for comparisons across them.