Calag Archive
Calag Archive
Biomarkers aid understanding of aquatic organism responses to environmental stressors
Publication Information
California Agriculture 57(4):110-115. https://doi.org/10.3733/ca.v057n04p110
Published October 01, 2003
PDF | Citation | Permissions
Abstract
Biomarkers can be useful tools for understanding the complex interactions that govern organism responses to environmental stressors and their sublethal effects on organism health. We conducted studies on two types of biomarkers: stress proteins and tissue alterations. A study on the freshwater fish medaka demonstrates that the ability to increase cellular stress-protein concentrations at specific life stages can be vitally important for normal embryo development. A field study on Asian clam investigates the usefulness of stress proteins and histopathology as indicators of exposure to and sublethal effects of environmental stressors in the northern San Francisco Bay and Delta.
Full text
Exposure to environmental stressors can result in biochemical, physiological and histological (tissue) alterations in living organisms. The presence of these alterations may serve as “biomarkers,” signaling exposure to stressors or adverse effects, which can range from molecular, cellular and tissue damage to genetic alterations. In the aquatic environment, such stressors can constitute changes in physical parameters such as temperature, pH or salinity, as well as toxic concentrations of chemical pollutants or any combination of these.
In the Sacramento-San Joaquin River watershed, populations of finfish are in decline (Bennett and Moyle 1996), and numerous aquatic species are either listed or proposed for listing as threatened or endangered. These include Sacramento splittail (Pogonichthys macrolepidotus), delta smelt (Hypomesus transpacificus), spring- and winter-run chinook salmon (Oncorhynchus tshaw-ytscha), coho salmon (Oncorhyn-chus kisutch) and steelhead trout (Oncorhynchus mykiss). Fisheries experts agree that survival of juvenile fish is a critical factor in maintaining healthy fish populations. Living in an environment that has been altered considerably by human activities, fish are often exposed to a multitude of stressors. Land-use patterns and regulated river flows have changed the temperature, flow and salinity in watersheds (Bennett and Moyle 1996). In addition, toxic contaminants and nutrients enter the water via agricultural and urban runoff, discharges from abandoned mines and point-source dischargers (U.S. Geological Survey 1998; Werner, Deanovic et al. 2000; Roth et al. 2001).
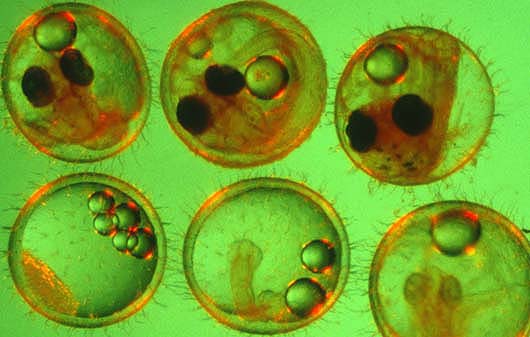
To better understand how stress proteins affect fish, medaka embryos at various stages of development, above, were subjected to heat shock. Stress proteins can serve as biomarkers, indicating exposure to environmental stresses such as temperature, pH, chemical pollution or salinity. Clockwise from upper left: stages 11, 19, 23, 26, 28 and 35.
Glossary
Glossary
Biomarkers:: Biochemical, physiological or histological indicators of either exposure to or effects of physical stressors or xenobiotic chemicals at the suborganismal or organismal level (Huggett et al. 1992).
Histopathology:: The study of lesions (alterations) in certain tissue types, including necrosis, inflammation and degeneration (Huggett et al. 1992).
Stress proteins/heat-shock proteins [hsp]:: A family of proteins that protect organisms from environmentally induced cellular damage. Members of the hsp70 protein family are involved in the stabilization of unfolded protein precursors, translocation of proteins across cellular membranes, dissolution of protein aggregates, and repair or degradation of damaged proteins. Several hsp70 proteins are always present in cells, while others are only produced in response to stressful environmental conditions (Feige et al. 1996).
The ability to protect cells at specific life stages can be vitally important for the normal development of organisms. Knowing the course of development, or ontogeny, of biomarkers shown to be important in adult stress responses, and their importance for normal embryo development, can improve understanding of the specific needs of these organisms for successful recruitment. Some biomarkers, such as the activity of enzymes like acetylcholine esterase or the cellular concentrations of metal-binding proteins (such as metallo-thioneins), enable scientists to determine if exposure to particular environmental stressors has triggered biochemical and cellular responses in the organism.
Such responses allow preliminary assessments of the impact and bio-availability of a specific type of stressor, but presently cannot provide specific information on higher effects such as survival or reproduction. Other biomarkers, such as cellular stress-protein levels, indicate that potentially harmful effects have occurred inside cells, and that cellular repair mechanisms have been activated. Finally, tissue damage can indicate effects at the organ level and provide insight into the organism's health. These higher-tier biomarkers are often less stressor-specific, but can reflect the combined effects of multiple stressors. On the other hand, they are often difficult to interpret, especially when applied individually in field studies. To reduce variability in field studies, cause-effect relationships between biomarkers and stressors are best examined using sessile organisms, such as shellfish, which do not move around and reflect conditions at a specific location. However, multiple stressor effects in the field can slow attempts to interpret biomarker responses. We conducted several studies in the field and laboratory to further elucidate the use of biomarkers as indicators of exposure to environmental stressors in the northern San Francisco Bay and Delta.
Stress proteins protect medaka
To date, the linkage between the age-dependent sensitivity of aquatic organisms to stressors and cellular stress responses is poorly understood. UC Davis researchers (Marty et al. 1990; Hamm et al. 1998) have determined that the sensitivity of fish embryos to toxic compounds is age-dependent and not related to differences in uptake rate or bioavailability, which determines how much of the chemical is taken up by the organism. Often this sensitivity is a function of the organism's ability to metabolize the chemical or activate cellular stress responses and repair mechanisms. The stress-protein response is among the mechanisms that protect organisms from environmentally induced cellular damage. Presence or absence of these protective proteins at a given developmental stage may influence how the organism is affected by exposure to physical environmental changes and/or toxic chemicals.
To elucidate the protective role of stress proteins in the embryonic development of fish, we characterized the response of the hsp70 protein family to heat-shock in several developmental stages of the freshwater fish medaka (Oryzias latipes)(Werner, Koger et al. 2001). Medaka are small, easily cultured fish that produce eggs year-round. Their continuous production and availability of eggs, transparent chorion (eggshell) and relatively small adult size facilitate studies of reproductive and developmental effects.
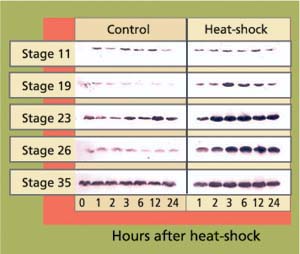
Embryos were obtained from a medaka colony continuously maintained at UC Davis since 1986. At each of five developmental stages, embryos (n = 100 per stage) were given a 30-minute heat-shock at 104°F and returned to culture conditions. Control embryos were maintained at 77°F. Samples of 10 heat-shocked and 10 control embryos were then taken for hsp analysis at 1,2,3,6,12 and 24 hours after heat-shock. Proteins were analyzed by gel electrophoresis and immunoblotting using specific antibodies that detect hsp70 (Affinity Bioreagents, Golden, Col.)(fig. 1). Hsp70 proteins were visualized using a chemoluminescent reagent, and subsequently measured by densitometry. For the study of developmental effects and hatching success, 50 embryos per stage were heat-shocked as described, then maintained at culture conditions until hatching. Development and viability were recorded daily.
Fig. 1. Western blots of stress protein hsp70 in heat-shocked (30 minutes, 104°F) and control embryos at different stages of development. Each band represents one pooled sample of five embryos. Band intensity reflects cellular concentrations of hsp70 proteins.
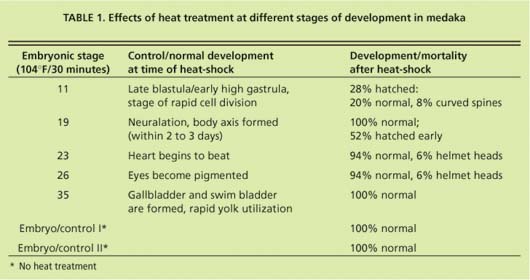
In early-stage embryos (stage 11 = early gastrula), stress-protein (hsp70) concentrations did not increase in response to heat-shock (fig. 13), while hsp70 proteins were induced in all other developmental stages. These early-stage embryos were considerably less tolerant to heat stress than embryos at stage 19 and older (table 1). Of stage 11 embryos heat-shocked at 104∞F for 30 minutes, 22% died within 1 day. By day 3 another 22% died. By day 4 another 28% showed retarded development when compared to controls, with the formation of large, clear spaces around the heart and over the yolk sac. In addition, eyes were absent or fragmented. These alterations have also been described in medaka embryos exposed to various toxicants (Marty et al. 1990), and represent pericardial edema, yolk sac and peritoneal edema, and microphthalmia (reduced growth of the eyes resulting in small eyes or blindness), respectively. Only 28% developed normally.
Hatching began after 7 days, and by day 14, 93% of normally developed embryos had hatched. Of the hatched larvae, 23% had curved spines. Stage 19 embryos heat-shocked at 104°F for 30 minutes developed normally, but initiated hatching earlier than the embryos heat-shocked at later stages and the control embryos. Eighty percent hatched on day 7 of development whereas hatching had occurred in only 0% to 14% of all control embryo groups. By day 9, all embryos heat-shocked at stages 19 to 35 and control embryos hatched, and showed no sign of developmental lesions. However, a small percentage of stage 23 and stage 26 embryos showed “helmet heads,” which occurs when all but the head emerges from the chorion and this structure remains closely attached to the head.
The stress-protein response is one of the most important cellular mechanisms to prevent and repair the adverse effects of thermal stress, some chemical pollutants and disease (Feige et al. 1996). Aquatic organisms respond to temperature stress and toxicant exposure by increasing cellular concentrations of hsp70 and related stress proteins (Sanders 1993; Iwama et al. 1998). Among the chemicals shown to produce this response are dioxin (Soimasuo et al. 2001), heavy metals (Baumann et al. 1993) and pesticides (Werner and Nagel 1997). Cellular stress-protein concentrations also increase in response to bacterial and viral infections (Zuegel and Kaufmann 1999).
The inability of early (stage 11) embryos to increase cellular concentrations of stress proteins may render them more susceptible to a variety of environmental stressors during a critical phase of their life cycle. It is of crucial importance that environmental decision-makers are aware of this when setting water-quality criteria for the protection of aquatic life. Protecting only the juvenile or adult organisms may not guarantee the survival of populations.
Asian clam and environmental stress
The Asian clam, or Amur River corbula (Potamocorbula amurensis), was introduced via ship ballast water into San Francisco Bay in the mid-1980s. The species has since spread prolifically throughout the Bay and Sacramento-San Joaquin River Delta, increasing to a peak population density of more than 12,000 per square yard (Carlton et al. 1990). It has become the dominant species in northern San Francisco Bay (Nichols et al. 1990), where it is implicated as the possible cause of a change in the existing community structure, reducing food supplies for juvenile fish ( see p. 104 ) (Werner and Hollibaugh 1993; Kimmerer and Orsi 1996; Feyrer 2000).
Because of its abundance and wide distribution, UC Davis and the U.S. Geological Survey (USGS) have been studying Asian clam as an indicator species for pollutant effects in San Francisco Bay. Biomarkers of reduced health — including lowered condition indices and energy reserves, and elevated levels of metal-binding proteins — have been observed to increase from San Francisco Bay eastward toward the Sacramento-San Joaquin River Delta. These indicators were partially linked to heavy metals, particularly cadmium, measured in clam tissues (Brown and Luoma 1995; Teh et al. 1999).
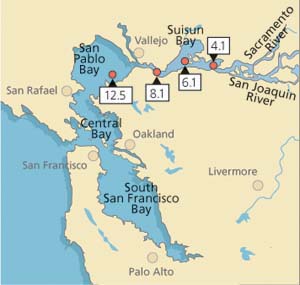
In this study, we attempted to link levels of stress proteins and tissue damage in field-collected Asian clam to concentrations of heavy metal in tissues (Werner and Hinton 1999, (2000). From July 1996 to January 1998, clams were sampled monthly (except October 1996, January to March 1997 and October 1997) from each of four stations in northern San Francisco Bay (fig. 2). Average salinities during the collection period ranged from 4.2 ± 4.0 parts per trillion (ppt) at station 4.1 (range: 0.06 to 15 ppt) to 22.3 ± 5.6 ppt at station 12.5 (range: 4.1 to 30 ppt).
Laboratory exposures to dissolved cadmium were conducted to validate results observed in the field and hsp70 proteins were analyzed as described previously. In Asian clam, the antibody used recognized two related proteins, hsp70 and hsp76. For an assessment of histological damage in reproductive organs and gills, clam tissues were scored for severity of pathological alterations as 0 = none, 1 = minimal, 2 = moderate and 3 = severe.
Stress proteins and tissue damage
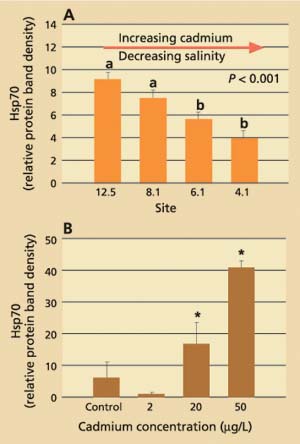
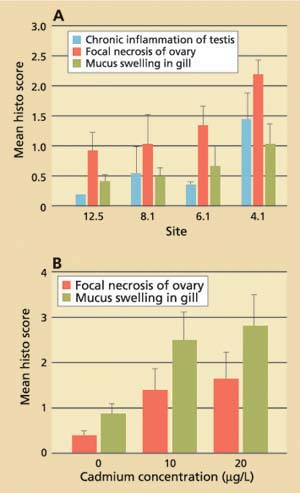
Data obtained for field-collected clams showed that tissue levels of hsp70 were significantly (P < 0.001) higher from stations with the lowest cadmium concentrations and highest salinities (12.5 and 8.1) than from stations with the highest cadmium concentrations and lowest salinities (4.1 and 6.1; fig. 3A). The laboratory results, on the other hand, showed that clams increase their cellular hsp70 levels with increasing cadmium concentrations (fig. 3B). Other heavy metals such as chromium-VI also induced hsp70 proteins. Histopathologic lesions in the testes (chronic inflammation with associated focal necrosis) were relatively common at station 4.1 (high cadmium, low salinity), especially during late spring and early summer (data not shown), and were rare at station 12.5 (low cadmium, high salinity) throughout the year (fig. 4A). Focal necrosis of the ovary and mucus-cell swelling in gills followed a similar pattern. A 14-day laboratory exposure of clams to dissolved cadmium caused increased lesion scores in the gills and ovaries (fig. 4B).
Contrary to our expectations, stress-protein levels, which increased with rising cadmium concentrations in our laboratory experiment, were lowest in clams from station 4.1 (high cadmium, low salinity) and highest at station 12.5 (low cadmium, high salinity), while physiological indicators of reduced health such as condition index (Teh et al. 1999) and the severity of tissue damage (Clark et al. 2000) suggested that the health of clams at station 4.1 was compromised. In addition, cadmium tissue concentrations and levels of metalbinding proteins were highest in clams from this station (Brown and Luoma 1995; R.C. Kaufman, UC Davis, personal communication), and reflected the increased heavy-metal concentrations at station 4.1.
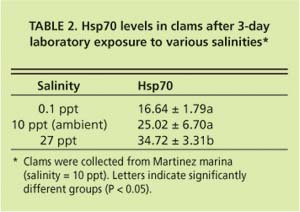
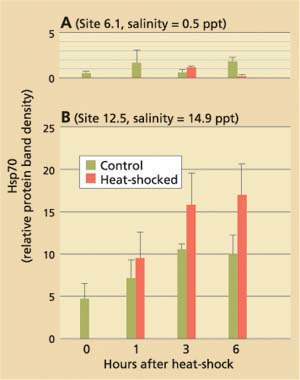
Current hypotheses to explain the apparent discrepancy in the clams' bio-marker responses focus on salinity as a stress factor, but do not exclude the possible influence of chemical stressors. While P. amurensis can tolerate very low salinities (0.1 ppt), prolonged exposure to freshwater is lethal. At station 4.1, salinity is close to the species' lower tolerance limit, with extended periods of freshwater conditions during winter and spring. Our laboratory experiments suggest that there is a link between cellular stress-protein levels and salinity. For example, clams collected in December 1998 from a low-salinity station (0.1 ppt) were not able to raise stress-protein concentrations in response to heat-shock (fig. 5A), a normal response to temperature stress, whereas clams from stations with higher salinities (5.6 ppt and 14.9 ppt) could (fig. 5B). In addition, clams exposed to low salinity (0.1 ppt) in the laboratory had lower tissue concentrations of stress protein than clams exposed to medium (10 ppt) and high (27 ppt) salinities (table 2).
This is not easily explained. Asian clam is an osmo-conformer, which means they can rapidly (within 48 hours, R.C. Kaufman, UC Davis, personal communication) adapt to changes in salinity by increasing or decreasing intracellular concentrations of certain amino acids (alanine and glycine betaine). Hsp70s perform important cellular functions but there is no indication that they are involved in osmo-regulation, the process animals use to adapt to changes in salinity, or that their concentrations are diluted selectively during osmo-adaptation.
Fig. 3. (A) Average stress protein (hsp70) levels in Asian clam from four stations in northern San Francisco Bay. Six clams per site were collected monthly from July 1996 to January 1998. Group a is significantly (P < 0.001) different from group b. (B) Average stress-protein levels in Asian clam (n = 6) after exposure to dissolved cadmium in the laboratory.* indicates significant difference (P < 0.05) from control and 2 μg/L-exposed clams. Error bars represent standard errors of the mean.
A possible explanation could lie in the energetic cost of osmo-regulation, and the effect of energy depletion on the clam's ability to maintain proper cellular function. Osmo-regulation is very costly in terms of energy consumption. In clams from stations with the lowest salinities, concentrations of glycogen (a carbohydrate used by animals to store energy) were significantly reduced, and gradually increased toward stations with the highest salinities (C. Brown, USGS, Menlo Park, personal communication). Such a reduction in cellular energy reserves could compromise the clam's ability to increase cellular stress-protein concentrations in an effort to adapt to stress. This, in turn, may lead to higher susceptibility to other environmental stressors and reduced organism health. Protein synthesis and repair are energy-intensive processes. Roberts et al. (1997) estimated that the total cost of protein synthesis under nonstressful conditions constitutes 20% to 25% of the energy budget of the bay mussel (Mytilus edulis) and repair of one damaged protein molecule, which is what stress proteins do, requires as much as 100 ATP (the energy “currency” of cells) molecules. Research shows that energy-deprived cells are more heat-sensitive than controls, indicating that their normal heat-shock response may be disrupted, and establishing a link between reduced ATP levels and compromised stress-protein function (Feige et al. 1996).
Fig. 4. (A) Results of histopathologic screening in Asian clam from four stations in northern San Francisco Bay. Ten clams per site were collected monthly from July 1996 to June 1997. (B) Average histopathologic screening scores in Asian clam (n = 10) after exposure to dissolved cadmium in the laboratory. Error bars represent standard errors of the mean.
Fig. 5. Results of stress-protein (hsp70) analyses in Asian clam collected from northern San Francisco Bay stations in December 1998 axnd exposed to brief heat-shock (15 min., 98°F) in the laboratory. Salinities at collection sites were (A) 0.5 ppt (site 6.1) and (B) 14.9 ppt (site 12.5). Clams from site 6.1 were unable to respond to heat-shock by increasing their cellular hsp70 concentrations.
The nonnative Asian clam was introduced into San Francisco Bay in the mid-1980s. Clams were collected from various sites in the Delta and tested for stress proteins and tissue damage. Tissue damage was common at sites where heavy metal concentrations were highest and stress proteins lowest, potentially signaling an impairment of reproductive capacity and cellular protective responses.
Consequences for organisms
Despite these complex interactions, which we are still striving to understand, our results to date show that biomarkers can be useful indicators of sublethal effects or responses to environmental stressors, and that the failure to raise cellular stress responses can have severe consequences for the survival and health of organisms. Our medaka study demonstrated that early-stage fish embryos are more sensitive to certain environmental stressors because they lack the ability to raise cellular concentrations of certain stress proteins. Our study on clams showed that histopathology is a sensitive indicator of compromised organism health. We also learned that the ability to raise cellular stress responses can be compromised when organisms experience prolonged exposure to environmentally stressful conditions such as low salinity. It is possible that the high degree of osmo-regulation required at very low salinities depleted energy reserves and, in turn, rendered clams more susceptible to additional stressors such as heavy metals.
If this concept can be transferred to other species and stressors, these kinds of studies will improve our understanding about the mechanisms underlying the effects of multiple stressors on organisms. However, there is still a paucity of information on the complex interactions and mechanisms that govern biomarker responses. This lack of thorough functional databases can render the interpretation of results from field studies difficult, especially where results need to be linked to organism or population health. Nevertheless, biomarkers offer valuable mechanistic insights into sublethal physiological processes that will eventually elucidate the complex links between environmental conditions, stress responses, and organism and population health.