Calag Archive
Calag Archive
Well-functioning cell mitochondria promote good health
Publication Information
California Agriculture 65(3):136-140. https://doi.org/10.3733/ca.v065n03p136
Published July 01, 2011
PDF | Citation | Permissions
Abstract
Mitochondriol function can be directly linked to protection from certain chronic diseases and conditions, such as heart disease, diabetes, metabolic syndrome and chronic inflammation, as well as the aging processes. Mitochondria are central to normal glucose, amino acid and fatty acid metabolism, in addition to antioxidant modulation and virtually all aspects of cell turnover and maintenance. Nutrition plays an essential role in optimizing such functions. We describe strategies for the regulation of mitochondria, as well as metabolic strategies for dealing with the thousands of compounds in plants and animal tissues that are metabolically important. Many of these compounds function to signal the up- or downregulation of mitochondria or act as antioxidants.
Full text
Editor's note: This article describes important functions and characteristics of mitochondria. It is the basis for the next article, “Biofactors in food promote health by enhancing mitochondrial function” ( page 141 ), which describes the actions of specific food components as cellular signals for mitochondriogenesis.
Aging and many chronic diseases and conditions such as heart disease, diabetes, metabolic syndrome and inflammation are affected by mitochondria, organelles that break down nutrients and produce most of the energy in our cells. These organelles act as the cell's chief metabolic control center, converting substances from the foods we eat into energy for essential functions. To generate the adenosine triphosphate (ATP) that powers most of the chemical reactions in cells, mitochondria use oxygen to help break down (metabolize) glucose, amino acids and fatty acids. Because this process requires oxygen, it is called cellular respiration. The control of oxygen use and respiration is central to normal growth and development, affecting virtually all aspects of the metabolism of glucose, amino acids and fatty acids and the modulation of reactive oxygen species (ROS) that can damage cells (Lane 2006).
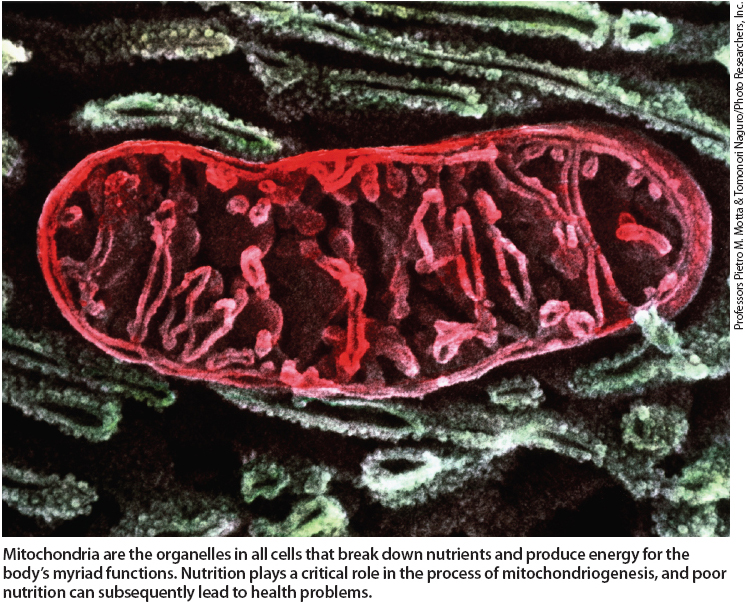
Mitochondria are the organelles in all cells that breakdown nutrients and produce energy for the body's myriad functions. Nutrition plays a critical role in the process of mitochondriogenesis, and poor nutrition can subsequently lead to health problems.
Mitochondria regulate these processes with great precision. For example, consider that over the course of a year, whether slender or obese, each of us consumes hundreds of pounds of food, yet our body weight usually does not vary more than a pound. Mitochondria are also key to regulating body temperature: the human production and use of ATP is equivalent to about 10,000 times more heat per day than that produced by an equal mass of the sun. Mitochondrial control of ATP production helps keep us from boiling in our own juices or even raising our body temperature a few degrees, except on rare occasions (Lane 2006; Scheffler 2007).
Regulation of mitochondria
The mechanisms for mitochondrial regulation include (1) changes in the number of mitochondria per cell, (2) control of the assembly and disassembly of mitochondria (turnover), (3) changes in the size and surface area of mitochondria and (4) regulation of the numerous catalytic steps important to the control of body fuel utilization, heat and ATP production. Besides the nucleus of the cell, mitochondria are the only cellular organelle in animals that contains a distinct set of genes as well as the machinery to manufacture some of its own messenger RNAs and proteins. Of the approximately 25,000 genes that make up the human genome, about 0.1% are found exclusively in the mitochondria (Scheffler 2007).
Indeed, mitochondrial DNA (mtDNA) has a separate evolutionary origin, being derived from the circular genomes of bacteria that were engulfed by early ancestors of the eukaryotic cells in today's plants and animals. These mitochondrial genes, plus another thousand or so in the nucleus (about 4% of the total nuclear genes), are associated with mitochondrial assembly and production. It is important to note that in most multicellular organisms, mtDNA is maternally inherited. This is partly because an egg contains 100,000 to one million mtDNA molecules, whereas a sperm cell contains only 100 to 1,000, and most of these are lost or degraded after fertilization.
Mitochondrial function and health
In addition to being key to the control of energy production and utilization, mitochondria are important to cellular regulatory signaling, cellular differentiation, apoptosis (programmed cell death or cellular turnover) and, when appropriate, cell replacement. The lifespan of a cell is directly linked to mitochondrial assembly and production (Spierings et al. 2005). Apoptosis occurs as part of the normal development, maintenance and renewal of tissue. In the average adult, between 50 billion and 70 billion cells turn over each day due to apoptosis (Lane 2006); this amounts to the proliferation and subsequent destruction of a mass of cells equal to one's body weight in a single year!
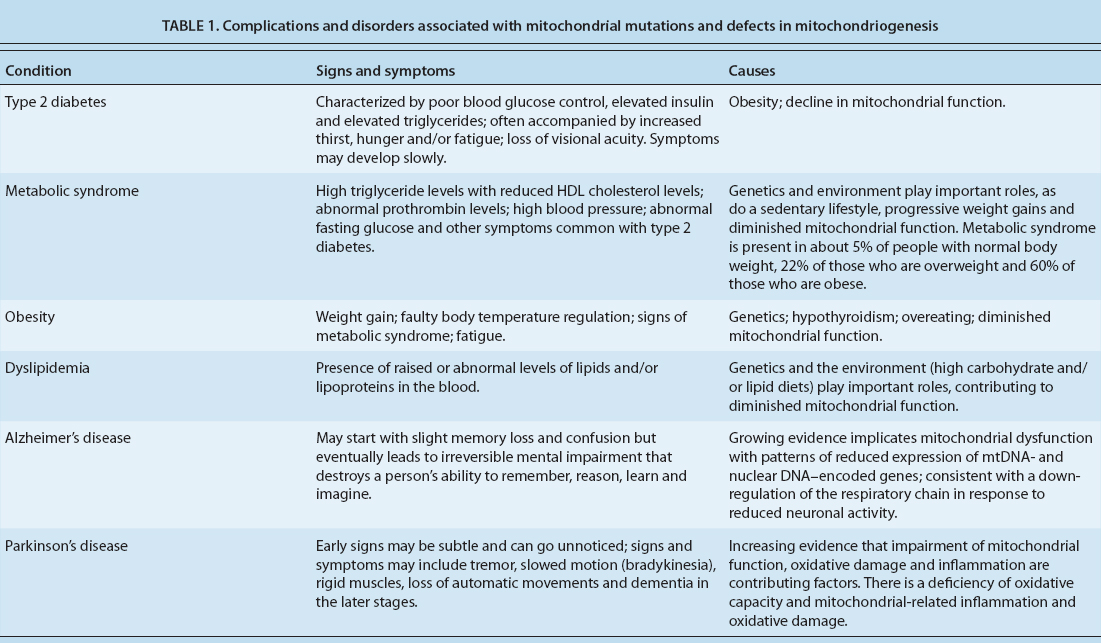
Consequently, when mitochondrial function is compromised at any level, a number of metabolic changes with health-related consequences can occur (table 1). In many cases, their direct causes are genetic in nature. However, the decline in mitochondrial efficiency with aging is thought to be a major contributor to metabolic syndrome, as well as neurological and psychiatric disorders such as Parkinson's and Alzheimer's disease (Guarente 2008). Understanding nongenetic factors such as diet and exercise is essential to correctly interpreting the health-related consequences of mitochondrial function.
TABLE 1. Complications and disorders associated with mitochondrial mutations and defects in mitochondriogenesis
Exercise intolerance (fatigue or breathlessness during exercise) is a common symptom of mitochondrial defects that are genetic as well as some of those that are due to normal aging (Tarnopolsky and Raha 2005). Such defects compromise the transfer of food energy into metabolic energy. This in turn reduces oxygen extraction by tissues and can result in the excessive generation of lactate and enhance the free radical production that may cause tissue damage.
Glossary
Adenosine triphosphate (ATP): A multifunctional nucleotide in cells often designated the “molecular unit of currency” of cellular energy transfer. When energy is derived from the breakdown of chemical bonds in food, a part of that energy goes into forming ATP, which is then used to drive reactions important to cellular and mechanical work (e.g., walking or running).
Antioxidant: A substance capable of inhibiting the oxidation of other molecules, particularly cellular components such as lipids, proteins and nucleic acids.
Apoptosis: The process of programmed cell death, in which the cell uses specialized cellular machinery to kill itself, so as to control cell numbers and eliminate cells that threaten the organism's survival.
Biofactor: Any material that has some level of biochemical function. The term is used as a general designation for functional chemicals, usually found in natural products, that contain, for example, vitamins, antioxidants or cell signaling agents.
Cell signaling: The relaying of molecular signals (chemical or physical, such as light) from a cell's exterior to its intracellular response elements and signaling molecules.
Messenger RNA (mRNA): Messenger ribonucleic acid, a molecule that encodes the chemical “blueprint” contained in genetic DNA, transcribing and carrying it to the site of protein synthesis to form a protein.
Metabolism (aerobic, anaerobic): The physical and chemical processes in an organism by which compounds are produced (anabolically formed), maintained (homeostatically maintained) or destroyed (catabolically altered). Energy for metabolic and mechanical work is derived from metabolism. Aerobic metabolism requires oxygen; anaerobic metabolism occurs in the absence of oxygen.
Mitochondria: Membrane-enclosed compartments (or organelles) found in most eukaryotic cells. They produce most of the cell's chemical energy, but as byproducts of this function, also generate significant amounts of damaging substances termed reactive oxygen species (ROS).
Mitochondriogenesis (mitochondrial biogenesis): The production of new mitochondria. This process can occur independently of normal cell division.
Phytoalexin: A substance (phytochemical) that is produced by a plant in response to bacterial or fungal pathogen attack. A number of substances that plants produce and utilize as phytoalexins are ingested by animals and function as antioxidants.
Reactive oxygen species (ROS): Chemically reactive, oxygen-containing molecules, most of which have unpaired electrons. ROS form as natural byproducts of normal oxygen metabolism and have important roles in cell signaling. ROS levels can also increase dramatically and produce significant damage to cell structures.
Respiration, cellular: The combined metabolic processes that are directed at capturing chemical energy from food and converting it to compounds, such as ATP, for eventual transfer to use in work-related functions (e.g., biosynthesis, locomotion or transport).
Transcriptional coactivator: A cofactor protein that stimulates mRNA transcription by binding at particular sites on DNA.
Xenobiotic metabolism: Sets of metabolic pathways that modify the chemical structure of xenobiotics — compounds foreign to an organism's normal biochemistry such as those produced by plants (phytochemicals), synthetic drugs and poisons. The same xenobiotic pathways are also important for the transformation and metabolism of certain vitamins, hormones and endogenous compounds important to integrated metabolism and cellular regulation.
Apoptosis.
Mitochondrial-related apoptosis is often accelerated in response to inflammation or protection from abnormal cell proliferation (such as that associated with some types of cancer) (Mann et al. 2005). The presence of abnormally elevated mitochondrial DNA in serum can be diagnostic for certain cancers because of apoptosis (Ellinger et al. 2008). Although here we specifically focus on the cells that control ATP regulation and use (that is, those in muscles and the liver, and those that are important to nerve transmissions), the good news is that many biofactors that improve cellular energy relationships — such as the enhancement of lipid and glucose metabolism — also aid in improving inflammation and immune responses (Mann et al. 2005).
Cell signaling.
Part of a complex system of communication, cell signaling helps govern the fundamental activities of cells (Gomperts et al. 2003). Errors in cellular information processing or signaling can be responsible for obesity and numerous diseases, such as cancer and diabetes. Higher numbers of mitochondria are often associated with improved function in neural and energy processing cells, such as liver and muscle cells.
Mitochondrial assembly.
In cells primarily designed for energy utilization and nutrient processing, the first steps of mitochondriogenesis often involve cell surface receptors responding to extracellular signals. These signals can come to a given cell in the form of both internal (e.g., hormones and cell-derived factors from other or adjacent cells) or external messenger molecules (e.g., dietary factors) that interact with the receptors of the given or targeted cell. Examples of internal messenger molecules include cytokines, hormones, growth factors, neurotransmitters and adhesion molecules that aid in cell-to-cell communication. External messengers include many bioactive factors (see page 141 ) (Rice-Evans and Packer 2003).
In some cases, cell surface receptors communicate with other cellular proteins, ranging from membrane proteins that open and close ion channels in the cell membrane to cellular enzymes that act as on/off switches by chemically activating proteins important to signaling pathways. Examples of these enzymes include kinases and phosphatases that add or remove phosphate groups from specific signaling molecules, thereby changing their chemical characteristics and in turn the levels of signaling activity. Such complex signaling pathways provide opportunities for feedback, signal amplification and interactions with other signals and signaling pathways.
The master regulators of mitochondriogenesis are a family of proteins that control the transcription of messenger RNAs, which in turn control and regulate the production of proteins needed to manufacture mitochondria. These proteins are called the peroxisome proliferator-activated receptor (PPAR) gamma family of transcriptional coactivators, and include one called PGC-1$$alpha$$, which enhances the expression of intermediate transcription factors that are important to mitochondriogenesis (Handschin and Spiegelman 2006). These factors include nuclear respiratory factors (NRFs), PPARs and TFAM (transcription factor A, mitochondrial). PPARs and NRFs play essential roles in the integration and regulation of cellular differentiation and metabolism, and TFAM is a key activator of mitochondrial DNA gene transcription.
Other factors regulated by PGC-1$$alpha$$ include CREB (cAMP response element binding) and STAT (signal transducer and activator of transcription). These transcription factors are important to mitochondriogenesis and can help coordinate mitochondrial function with the dozens of other processes important to cell growth and maintenance (Gough et al. 2009; Wegrzyn et al. 2009). In brief, these factors work together either by enhancing the binding of one or more factors or by decreasing their binding affinities, thereby modulating the production of messenger RNAs involved in mitochondrial assembly and disassembly.
Mitochondrial disassembly.
Like any highly responsive and dynamic process, mitochondrial production must be coordinated with disassembly as necessary. Mitochondrial disassembly is mediated by cell-surface receptors in the Fas family of proteins that modulate apoptosis. These proteins are closely related to other apoptotic proteins, the tumor necrosis factor and the nerve growth factor receptor family of proteins (Nagata and Golstein 1995). When Fas proteins are activated, they in turn activate and mobilize other protein families involved in apoptosis, such as the Bcl-2 family. Bcl-2 proteins can either promote or inhibit apoptosis (Spierings et al. 2005; Wong and Puthalakath 2008). One of the best-understood Bcl-2 proteins is called BAX, which promotes apoptosis by competing with another Bcl-2 protein (called Bcl-2 proper) that inhibits apoptosis (Lane 2006; Wong and Puthalakath 2008). BAX inserts itself primarily into the outer mitochondrial membrane and induces the opening of voltage-dependent channels, which results in the release of proteins such as cytochrome c (an important mitochondrial respiratory protein) and other pro-apoptotic factors.
In addition, Bcl-2 proteins can activate any of a large family of caspase enzymes, which break down proteins and can degrade and modify various cellular components. The activation of caspases plays a role in both apoptosis and normal development. It is important to note that in some cells, caspases are required for normal immune and inflammatory responses. Consequently, the failure to control apoptosis is one of the main contributors to uncontrolled cancer proliferation and tumor development, as well as the expression of certain autoimmune diseases.
Moreover, to add to this complex arrangement, a family of enzymes in the nucleus also affects apoptosis (Alcain and Villalba 2009; Elliott and Jirousek 2008; Lane 2006; Sharman 2010). Called silent information regulators (or sirtuins), these enzymes modify histones, which are proteins in the nucleus that serve as the scaffolding that helps organize nuclear DNA. When histones are modified, different segments of DNA can be exposed and transcribed into mRNA. These sirtuins seem particularly important to the exposure of genes related to apoptosis control.
Other effects on mitochondriogenesis
Mitochondriogenesis and signaling are significantly affected by two other factors: (1) xenobiotic metabolism, which evolved mainly to aid in the disposal and transformation of hundreds of plant pigments in the diet and (2) ROS, which include free radicals and other chemically reactive oxygen-containing molecules.
Xenobiotic metabolism.
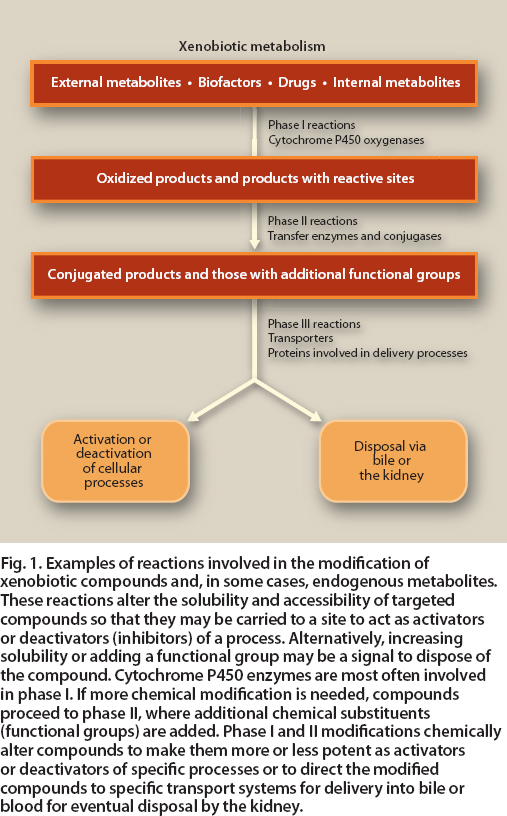
Besides being critical to phytochemical availability and disposal, xenobiotic metabolism plays a role in activating many of the biofactors in food that act as cell signaling agents (Johnson 2008) (fig. 1). Although the strategic steps involved in xenobiotic metabolism are relatively few, an extraordinary aspect is its ability to tactically modify thousands of compounds with differing chemical properties for disposal and use.
Fig. 1. Examples of reactions involved in the modification of xenobiotic compounds and, in some cases, endogenous metabolites. These reactions alter the solubility and accessibility of targeted compounds so that they may be carried to a site to act as activators or deactivators (inhibitors) of a process. Alternatively, increasing solubility or adding a functional group may be a signal to dispose of the compound. Cytochrome P450 enzymes are most often involved in phase I. If more chemical modification is needed, compounds proceed to phase II, where additional chemical substituents (functional groups) are added. Phase I and II modifications chemically alter compounds to make them more or less potent as activators or deactivators of specific processes or to direct the modified compounds to specific transport systems for delivery into bile or blood for eventual disposal by the kidney.
Xenobiotic metabolism is divided into three phases. In phase I, enzymes (cytochrome P450 oxidases) catalyze new reactive or polar groups on compounds that range from substances created by the body's cells to dietary factors to xenobiotics. In phase II, transferase enzymes catalyze the conjugation of the phase I products to other compounds, which results in additional novel or unique properties. In phase III, these modified products are recognized by cellular efflux transporters and may be pumped out of cells for disposal via the kidney or bile. However, along the way some of these compounds can take on novel biological activities (see page 141 ).
Reactive oxygen species.
ROS are products of normal metabolism, such as aerobic metabolism in mitochondria, and environmental stresses, such as excess exposure to ultraviolet light. ROS are important to many aspects of normal metabolism; for example, cells involved in the destruction of foreign substances may use ROS as a part of their arsenal (Bonekamp et al. 2009). However, excess ROS can be highly destructive to cell membranes, DNA and proteins, and can lead to the destruction of mitochondria and apoptosis if not controlled.
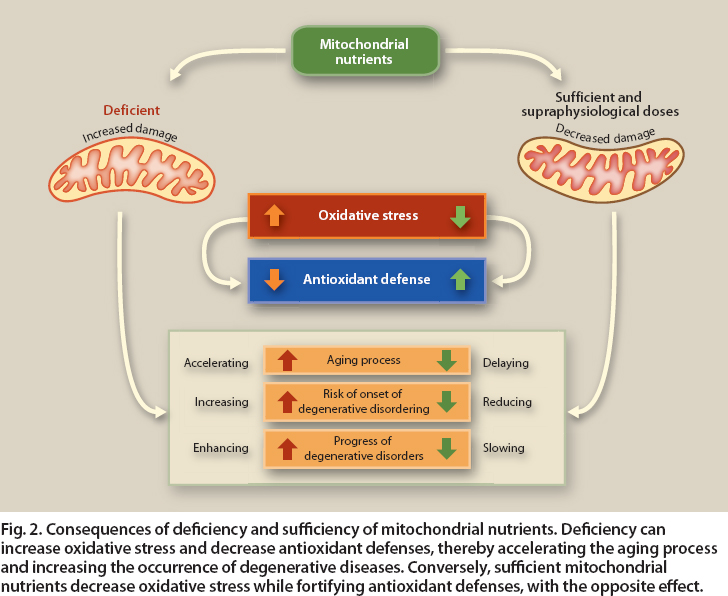
Phytochemicals can help protect against ROS, for example by inducing enzymes that reduce free radicals. In addition, when damage is severe, phytochemicals can induce signaling proteins such as Bcl-2 that lead to the turnover of damaged mitochondria and apoptosis of their associated cells (Bonekamp et al. 2009; Shay and Banz 2005) (fig. 2).
Fig. 2. Consequences of deficiency and sufficiency of mitochondrial nutrients. Deficiency can increase oxidative stress and decrease antioxidant defenses, thereby accelerating the aging process and increasing the occurrence of degenerative diseases. Conversely, sufficient mitochondrial nutrients decrease oxidative stress while fortifying antioxidant defenses, with the opposite effect.
Mitochondrial medicine
Mitochondria are responsible for most aerobic oxidative metabolic functions and the conversion of substances in the foods we eat into energy. The discovery of links between mitochondria and diseases has led to a new field of study called mitochondrial medicine. More than 40 genetic diseases are now recognized as involving mitochondria directly, affecting an estimated 1 out of 4,000 individuals. In addition, mitochondria also play a role in metabolic syndrome, obesity and diabetes, major chronic diseases affecting one-third to one-half of Western populations. A common feature of these diseases is that mitochondria, whether genetically defective or abnormally reduced in number, are unable to completely burn food and oxygen to generate energy. This in turn results in impaired oxygen utilization, which can lead to an increase in damaging ROS as side products as well as other toxic metabolic products (such as ammonia, which may not be efficiently incorporated into urea by metabolic pathways located in mitochondria) (fig. 2).
To understand exactly how phytochemicals may help protect against mitochondrial-related diseases, it is important to appreciate that highly regulated complex pathways have evolved to aid in their nutritional availability, disposal, and in some cases, biological activation. According to the free-radical theory, oxidative damage initiated by ROS is a major contributor to the functional declines associated with aging and many diseases. In this regard, many biofactors can act both in mitochondrial signaling and as ROS scavengers (see page 141 ). This exciting area of nutrition research provides evidence that California agriculture can play a beneficial role in oxidative metabolism, thus promoting good health.