- Author: Cris L. Johnson
Asian Citrus Psyllid and Huanglongbing
Trying to stay abreast of the insect and disease it carries to citrus?
The Ventura County Farm Bureau and Ventura County ACP-HLB Taks Force have put together links and a Facebook page that have all the latest breaking news concerning this threat to California's citrus industry and to the iconic backyard tree.
This is one of the best ways to find out more about this pest/disease complex and how this threat is being addressed.
- Author: Elizabeth Fichtner and Rachel Elkins
Lime-induced Iron Chlorosis: a nutritional challenge in the culture of several subtropical perennial crops in California
Elizabeth Fichtner, UCCE Tulare County and Rachel Elkins, UCCE Lake and Mendocino Counties
Spring, and new leaves are coming out, but this could, but yellow could be a sign of iron chlorosis, as well.
Although iron (Fe) is the 4th most abundant element in the lithosphere, Fe deficiency is among the most common plant micronutrient deficiencies. Fe deficiency in plants is common in calcareous soils, waterlogged soils, sandy soils low in total Fe, and in peat and muck soils where organic matter chelates Fe, rendering the element unavailable for plant uptake. In California, lime-induced Fe deficiency is often observed in soils and irrigation water containing free lime, and is exacerbated by conditions that impede soil drainage (ie. compaction, high clay content), resulting in reductive conditions. Given that over 30% of the world's soils are calcareous, lime-induced Fe deficiency is a challenge in numerous perennial cropping systems including: grapes, pears, apple, citrus, avocado, pecans, and stone fruit (prune, almond, apricot, peach, nectarine, cherry).
In most soils, Fe oxides are the common source of Fe for plant nutrition. Solubility of Fe oxides is pH dependant; as pH increases, the free ionic forms of the micronutrient are changed to the hydroxy ions, and finally to the insoluble hydroxides or oxides. In calcareous soils, the bicarbonate ion inhibits mobilization of accumulated Fe from roots to foliage and directly affects availability of Fe in soil by buffering soil pH. When irrigation water is also high in bicarbonate, probability of Fe deficiency is enhanced because bicarbonate is continuously supplied to the soil, and more importantly, the roots may become crusted with lime as water evaporates, thus inhibiting root growth and function. Inside the plant, bicarbonate inhibits nutrient translocation from roots to aboveground plant parts. The adverse effects of high bicarbonate levels are exacerbated in very saturated, very dry, or compact soils, where bicarbonate levels increase concurrent with diminished root growth and nutrient uptake.
Symptoms of Fe deficiency in plants
Fe is immobile in plants; therefore, symptoms appear in young leaves. Interveinal chlorosis (Figure 1) is the main symptom associated with Fe deficiency, followed by reduced shoot and root growth, complete foliar chlorosis, defoliation, shoot dieback, and under severe conditions may result in tree mortality. Overall productivity (yield) is reduced, mainly from a reduced number of fruiting points.
Plant Adaptation
Plant species and cultivars vary in their sensitivity to Fe deficiency, and are categorized as either "Fe-efficient" or "Fe-inefficient". Fe-efficient plants have Fe uptake systems that are switched on under conditions of Fe deficiency. Fe-inefficient plants are unable to respond to Fe deficient conditions. All Fe-efficient plants, except grasses, utilize a Fe-uptake mechanism known as Strategy 1. Strategy 1 plants decrease rhizosphere pH by release of protons, thus increasing Fe solubility. Some plants may excrete organic compounds in the rhizosphere that reduce ferric iron (Fe3+) to the more soluble ferrous (Fe2+) forms or form soluble complexes that maintain Fe in solution. Additionally, roots of Strategy 1 plants have specialized mechanisms for reduction, uptake, and transfer of Fe within the plant. Strategy 2 plants (grasses) produce low molecular weight compounds called phytosiderophores which chelate Fe and take up the chelated Fe with a specific transport system.
Amelioration of Fe chlorosis
Planting sites in calcareous soils should be well drained to provide optimal conditions for root growth and nutrient uptake. Waterlogged and compact soils contain
more carbon dioxide, which reacts with lime to form even more bicarbonate. These conditions, as well as very dry soils, also inhibit microbial activity which aids in
solubilization and chelation of Fe. Prior to planting, soils and water should be tested to determine the pH, lime equivalent, and bicarbonate concentration. Bicarbonate concentrations greater than 3 meq/L in irrigation water increase the hazard of lime accumulation on and around roots. If high bicarbonate water must be used, the pH must be adjusted to 6.0-6.5 to dissolve the bicarbonate and prevent it from negating the effects of soil-based treatments. In microsprinker and drip systems, acidification of irrigation water will also reduce the risk of emitter clogging, a common problem at bicarbonate levels over 2 meq/L. The cost of reducing the pH of irrigation water will more than compensate for the savings incurred from avoiding wasted investment in failed soil- and plant-based remedies. Systems can be set up to continuously and safely inject water with acids such as sulfuric, urea-sulfuric, or phosphoric during irrigations. Specific choice and rate will depend on crop, soil type, other nutrient needs, availability, and cost. Downstream pH meters are available to continuously adjust rate of acid use. Acetic and citric acid can be utilized by organic growers.
Soil based pre-plant treatments to reduce pH include elemental sulfur (S) and acids as mentioned above. It is only necessary to treat a limited area near the root zone to ameliorate symptoms because the tree only needs to take up a small amount of Fe. Material can be shanked in or banded and incorporated in the prospective tree row. One ton of elemental sulfur per treated acre is needed to mitigate three tons of lime, and may need to be re-applied every 3 to 5 years after planting. The addition of organic matter such as well-composted manures will benefit poorly drained or compact soils by increasing aeration for better root growth, fostering chelation of nutrient cations, and reducing pH (depending on source material).
If possible, choose a Fe efficient species or cultivar. In perennial systems, lime-tolerant rootstocks may be the first line of defense in combating Fe deficiency. Some rootstocksmentioned are peach-almond and Krymsk-86 for stone fruit, Gisela 5 for cherry, and Pyrus communis for pear. Ongoing research studies in Europe focus on screening rootstocks of grape and olive for lime tolerance.
Once soil and water quality improvements are made, post-plant management strategies may also be implemented to ameliorate lime-induced Fe chlorosis in the short term. Soil can be acidified as described above. Individual trees can be treated by digging four to six 12-24 inch
holes around the drip line and burying a mixture of sulfur and Fe fertilizer. Historically, two principal methods have been utilized: 1) foliar application of inorganic Fe salts (ie. ferrous sulfate), and 2) soil or foliar application of synthetic chelates. Application of Fe salts to foliage may have mixed results due to limited penetration of Fe into leaves and inadequate mobilization within the plant. Use of Fe chelates may be of benefit; however, they are expensive and pose an environmental concern due to their mobility within the soil profile. Because soil lime interferes with Fe mobility with the plant, repeat application of inorganic Fe salts or Fe chelates may be necessary throughout the growing season.
Choice of nitrogen (N) fertilizer may also influence solubility of rhizosphere Fe. When N is applied in the ammonium form (NH4+), the root releases a proton (H+) to maintain a charge balance, thus reducing rhizosphere pH. Alternately, fertilization with nitrate (NO3-) results in root release of hydroxyl ions (OH-), resulting in an increase in rhizosphere pH. Solubility of Fe3+ increases 1000 fold with each one unit decrease in pH; therefore, fertility-induced rhizosphere pH changes may significantly influence Fe availability.
New methods for amelioration of Fe chlorosis are under investigation. For example, container studies have demonstrated that inter-planting sheep's fescue, a Strategy 2 plant, with a Fe-inefficient grape rootstock may ameliorate Fe chlorosis in grape. In this system, the grass produces a phytosiderophore that enhances Fe availability to the grape. Additionally, soil amendment with Fe3(PO4)2• 8H2O), a synthetic iron(II)-phosphate analogous to the mineral vivianite, has been effective at preventing Fe chlorosis in lemon, pear, olive, kiwi, and peach. Vivianite has a high Fe content (~30%) and serves as a slow release source of Fe in calcareous soils.
Figures below: 1) Shoot dieback in citrus, 2) Interveinal chlorosis in citrus and 3) Various stages of iron chlorosis in avocado.



- Author: Ben Faber
|
Invasive Ambrosia Beetle/Fusarium Complex |
|
You are invited to attend a public meeting about the invasive ambrosia beetle/Fusarium complex that are threatening avocado, oak, sycamore, persimmon, and box elder trees in California.
August 14, 2012 2:00 - 5:00 pm Marriott Hotel Riverside, CA
Preregistration is required but there is no fee to attend. There is, however, a $4.00 parking fee.
For more information and to view the flyer, please click here or contact Mary Lou Arpaia at mlarpaia@ucanr.edu or call (559) 288-8507. Below, picture of the cankers caused by the ambrosia beetle and where the insect starte growing the fungus which will eventually kill the tree.
|
Fusarium dieback picture
avocado fusarium
- Author: Brad Hanson
Today I thought I'd share a recent research report on the the phenomenon of "enhanced" degradation of the herbicide simazine in citrus orchard soils. Click here for a link to the publication in the open-source journal, Air, Soil, and Water Research (Abit et al. 2012. Air Soil and Water Research 5:69-78). The lead author was a UC Davis post doctoral researcher and her coauthors include UC Davis, USDA-ARS, Fresno State, and UC Cooperative Extension folks.
This work was started several years ago in response to some questions from San Joaquin Valley orchard and vineyardists poor weed control with simazine. They suspected herbicide resistance, which is certainly a possibility given the number of weed biotypes resistant to photosystem II inhibitors reported worldwide (69 biotypes at latest count according to http://www.weedscience.org). However, when we visited some of the fields, it just didn't look like resistance or poor applications - too many species affected, good early control but poor residual performance, affected some orchards but not others, no clear application problems. 
Some colleagues in Colorado and Mississippi were conducting research on faster-than-normal degradation of atrazine, a related triazine herbicide. We decided to conduct laboratory studies to determine if the poor residual performance in our orchard systems could also be due to the so-called "enhanced degradation".
First, some background. To a large degree, all herbicides degrade is soil - a complex molocule is eventually broken down into component elements; however the rate of degradation can vary greatly. The two biggest contributors to herbicide degradation are "chemical processes" that include all sorts of ways that chemical bonds are broken and "microbial processes" in which soil microorganisms use the molocule as a carbon source (ie "food"). Generally, microbial processes are the most important but are usually not specific to a particular herbicide. Sometimes, however, a microbial population with a special ability to degrade a particular herbicide (or chemically related compounds) can build up in the population and very rapidly degrade all of the herbicide. [analogy: a bowl of candy on your desk that lasts for weeks if you eat one or two per day but lasts only a few seconds when your hungry teenager and his friends show up!].
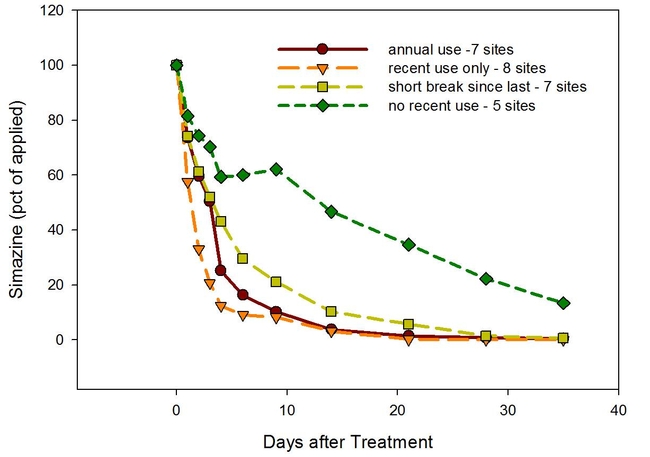
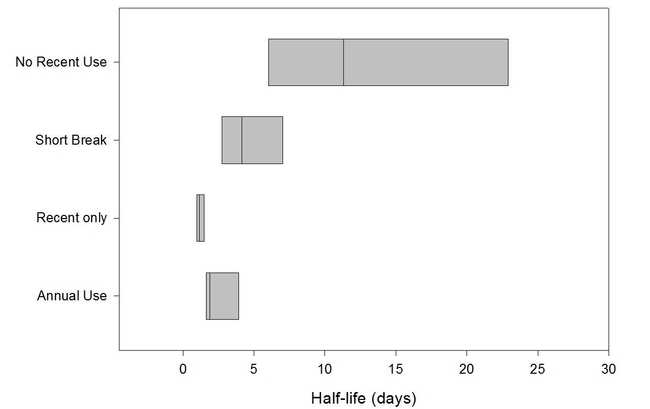
To determine if there were differences in simazine degradation rates among orchards, we collected soil from 27 citrus orchards in Tulare and Ventura counties. The orchards had a range of simazine use histories ranging from being treated every year for decades to having no simazine for at least the past 15 years.
1. In one set of experiments, we found that the soils that had not been exposed to simazine for at least 15 years degraded the herbicide much more slowly and that annual use (for the past 5 years, or longer) tended to be accociated with faster degradation.
2. In a second set of experiments, we autoclaved (heat sterilized) soil to kill any microbes present to determine if the herbicide degradation was due to microbial activity. This study showed that the degradation was almost completely due to microbial degradataion - simazine degradation was almost non-existent in autoclaved soils. Some soils have the the specialized microbial population while others do not.
The conclusions of the study were that repeated use of simazine can lead to more rapid degradation of the herbicide but that the correlation was not perfect. The correlations were better in the Tulare County sites than in the Ventura County soils. Some soils with long-term use of the herbicide continued to degrade simazine at a "normal" rate while other degraded it faster than predicted.
Overall, this is an interesting phenomenon that growers and pest control advisors should be aware of when making weed control decisions. Simazine can provide very effective and economical weed control in many situations but, as always, we recommend monitoring orchard conditions and adjusting weed control strategies accordingly.
This research was partially supported by funding from the California Citrus Research Board and USDA-ARS.
A couple of my previous posts on herbicide degradation at the UC Weed Science Blog can be found at: http://ucanr.org/blogs/blogcore/postdetail.cfm?postnum=5929 and http://ucanr.org/blogs/blogcore/postdetail.cfm?postnum=3923
- Author: Simon Newett
Thick, waxy coating on avocado leaves makes foliar nutrients difficult to abosorb.
LTTLE EVIDENCE TO SUPPORT THE USE OF FOLIAR APPLIED NUTRIENTS IN AVOCADO
Simon Newett, Extension Horticulturist.Department of Primary Industries and Fisheries, Maroochy Research Station, Mayers Road, Nambour 4560, Queensland, Australia. Previously published in: Talking Avocados (published by Avocados Australia Ltd), 11(2), 24-27.
Introduction
Foliar fertiliser application is sometimes promoted as an effective means of supplying nutrients to avocado. On the market are various products being promoted as foliar nutrients for avocado, some proponents even suggest that their products do away with the need for soil applied nutrients. This article briefly reviews the literature relating to foliar feeding of avocado and examines the anatomy of the avocado leaf and flower in relation to nutrient uptake.
The avocado leaf
The structure of plant leaves has evolved primarily to capture sunlight and exchange gases, roots have evolved to absorb nutrients and water and anchor the plant. Any absorption of nutrients by leaves is therefore likely to be more fortuitous than by design. In some crops passive nutrient absorption by leaves is occasionally sufficient to supplement the supply of nutrients taken up by the roots. Most often this involves trace elements, which as their name suggests are required in very small amounts (eg. copper and zinc). However if non-mobile elements or elements with limited mobility in the plant (eg. calcium, phosphorus, zinc, boron and iron) are absorbed when foliar sprayed they are not likely to make it down to the roots where they are also needed. Most nutrients will move freely in the water stream but the movement of many is restricted in the phloem, hence leaf applications don't meet the requirements of deficient trees. Occasionally major elements (such as nitrogen and potassium) are applied to make up for a temporary shortfall or provide a boost at a critical time. Citrus is an example of a crop where some benefits from foliar applied nutrients have been reported.
The ability of the leaf to absorb nutrients from its surface must depend to some degree on the permeability of its epidermis (outer layer) and the presence and density of stomates (pores for the exchange of gases). Scanning Electron Microscope studies of mature leaves and floral structures in avocado show the presence of a waxy layer on both the upper and lower surfaces of mature avocado leaves (Whiley et al, 1988). On the upper surface the wax appears as a continuous layer and there are no stomates. On the lower surface the wax layer is globular and stomates are present. Blanke and Lovatt (1993) describe the avocado leaf as having a dense outer wax cover in the form of rodlets on young leaves and dendritic (branching) crystals on old leaves including the guard cells (guard cells surround stomates). The flower petals and sepals in avocado have stomates on their lower surfaces and no wax layers on either surface, which might explain why floral sprays of boron might work.
Literature review
Nitrogen
Based upon total leaf nitrogen concentration, Embleton and Jones (unpublished) in a replicated trial in California in the early 1950's found no response to leaf sprays of urea on mature 'Fuerte' avocado trees in the field. Up to three sprays a year were applied.
Nevin et al (1990) reviewed urea foliar fertilisation of avocado and found only one study (Aziz et al., 1975) that reported positive results in terms of fruit yield. This trial by Aziz et al (1975) involved drenching sprays of significant amounts of urea four times a year (250 to 500 g of nitrogen per tree annually). It is unclear whether or not considerable amounts of the drenching spray reached the ground, nevertheless, the amounts applied were very high for foliar applications. No leaf analysis data was reported.
Galindo-Tovar (1983) was able to increase leaf nitrogen concentrations in ‘Hass’ avocado seedlings grown in a glasshouse with low concentrations of urea. However similar treatments on 3-year-old ‘Hass’ in the field for each month during spring failed to increase leaf nitrogen in mature leaves sampled a week after spraying. The author cited evidence for crops other than avocado suggesting that urea can penetrate leaf surfaces when grown in a greenhouse, but when grown in the field under full sun, leaf surfaces are different and resist movement of nitrogen into the leaf.
Klein & Zilkah (1986) reported substantial uptake of foliar urea-N when detached leaves of 'Fuerte' avocado were dipped in urea solutions. Zilkah et al (1987) reported the translocation of 15N from foliar-applied urea to vegetative and reproductive sinks of both 'Fuerte' and 'Hass' avocado. Despite the apparent response achieved by Aziz et al in Egypt, Klein & Zilkah, and Zilkah et al in Israel, attempts at the University of California to demonstrate significant uptake of nitrogen from foliar sprays have not been successful (Nevin et al., 1990).
Research at the University of California, Riverside, provided evidence that the leaf nitrogen content of 'Hass' avocado was not increased by foliar application of urea at the same concentration that increased citrus leaf nitrogen content two-fold (Nevin et al., 1990). Maximum uptake of 14C-urea by 'Hass' avocado leaves was physiologically insignificant after 2 days. Over 96% of the 14C-urea applied was recovered from the leaf surface even after 5 days. Maximum uptake of 14C-urea by leaves of 'Gwen' and 'Fuerte' was less than 7%. 15N, 14C-urea and 65Zn are radioactive forms of nitrogen, urea and zinc respectively that are used to track their movement through the plant.
Potassium
Sing and McNeil (1992) conducted a study on an orchard with a history of potassium deficiency where high magnesium levels in the soil competed with potassium for uptake. Foliar applications of 3.6% potassium nitrate were applied at half leaf expansion, full leaf expansion and one month after full leaf expansion. These foliar applications of potassium nitrate were effective in increasing the potassium level in the leaves of 'Hass' avocado trees, however two to three foliar applications per year were required to achieve the same result as one application of potassium sulphate (banded) to the soil once every 2 to 3 years. Accounting for labour and material costs the foliar sprays of potassium nitrate were estimated to be more expensive than soil applied potassium sulphate applied every three years. The foliar sprays also affected the levels of other nutrients in the leaf, some negatively.
Calcium
Calcium is receiving attention as an element in avocado fruit associated with better quality and longer shelf life. Several different calcium products were tested during the 1980’s as foliar sprays in South Africa in an attempt to raise fruit calcium levels but none were found to be effective.
Veldman (1983) reported that the treatment of avocado trees with one, three and six calcium nitrate sprays did not successfully control pulp spot in avocado fruit and there was no increase in fruit calcium levels on sprayed treatments.
Whiley et al (1997) report that calcium foliar sprays during fruit growth have little effect on internal concentrations in most fruit due to poor absorption by fruit, and lack of translocation within the tree.
Boron
Some benefits have been reported from foliar application of boron if applied at flowering. Timing is important because it appears that absorption takes place through flower structures and not leaves.
Jayanath and Lovatt (1995) reported on results of four bloom studies (two glasshouse and two field experiments) which demonstrated the efficacy of applying boron or urea sprays to 'Hass' avocado inflorescences during early expansion (cauliflower stage) but prior to full panicle expansion and anthesis. Anatomical analysis of the flowers provided evidence that the boron prebloom spray increased the number of pollen tubes that reached the ovule and also increased ovule viability, but to a lesser degree than urea. The urea prebloom spray increased ovule viability compared to boron-treated or untreated flowers. Urea also increased the number of pollen tubes that reached the ovule, but to a lesser degree than boron. However, combining boron and urea resulted in a negative effect even when the urea was applied 8 days after the boron. Lovatt (unpublished) provided an update on this work at the World Avocado Congress in 1999, after 3 years of field trials the only treatment to have a positive effect on pollination was the boron in Year 2, the most likely reason why it didn’t work in other years was thought to be low temperatures. There were only hardened leaves present at the time of foliar applications suggesting that uptake was through flower parts.
Whiley et al (1996) report that despite an increase in fruit set with foliar sprays of boron during flowering there has been no convincing evidence that showed increased final yield. Root growth has a requirement for boron and in deficient trees it is unlikely that sufficient nutrient from foliar applications would be translocated to the roots. Foliar applications have the advantage that specific organs can be targeted to enhance their boron concentrations, but with the disadvantage that insufficient boron can be absorbed through leaves to mediate chronic deficiency in trees. Soil applications have been shown to dramatically improve the health of boron deficient trees.
Mans (1996) experimented with ‘Hass’ trees that had leaf levels of nitrogen and boron below the accepted norms (N was 1.71% and B was 23ppm). The aim of this trial was to see if supplying nutrients directly on the flowers could increase the yield of ‘Hass’ trees growing in a cool environment. Mans (1996) found that if a multi-nutrient spray that included nitrogen and boron was applied as the first flowers started to open then he could increase yield and distribution of fruit size. The stage of flowering when spraying takes place was very important. Sprays that were applied pre-bloom, at fruitset or when fruitlets were present were not effective.
Iron
Kadman and Lahav (1971-1972) reported that the only means to control iron chlorosis in already established avocado orchards is soil application of iron chelates since applications of various iron compounds by foliar sprays have not been successful on a commercial scale. Gregoriou et al (1983) found that the quickest and most successful treatment of trees suffering from iron chlorosis on calcareous soils was obtained by incorporating Sequestrene 138 Fe-EDDHA in the soil.
Zinc
Kadman and Cohen (1977) found that avocado trees have difficulties in absorbing mineral elements through their foliage. In spite of this, spraying of apparently zinc-deficient orchards was rather common in California and some other countries. In Israel, some growers spray their orchards, but as experiments have shown, no apparent improvement occurs in leaves or fruits following such treatment. The results presented in this paper indicate that the penetration of zinc through the leaves is so slight that there is practically no benefit through supplying it by foliar sprays.
Zinc deficiency is common in avocado and is particularly difficult to address on high pH (alkaline) soils. Crowley et al (1996) evaluated methods for zinc fertilisation of ‘Hass’ avocado trees in a 2-year field experiment on a commercial orchard located on a calcareous soil (pH 7.8) in California. The fertilisation methods were:
• soil or irrigation-applied zinc sulphate
• irrigation-applied zinc chelate (Zn-EDTA)
• trunk injection of zinc nitrate
• foliar applications of zinc sulphate, zinc oxide, or zinc metalosate.
•
Among the three soil and irrigation treatments, zinc sulphate applied at 3.2 kg per tree either as a quarterly irrigation or annually as a soil application was the most effective and increased leaf tissue zinc concentrations to 75 and 90 mg/kg respectively. Experiments with 65Zn applied to leaves of greenhouse seedlings, showed that less than 1% of zinc applied as zinc sulphate or zinc metalosate was actually taken up by the leaf tissue. There was also little translocation of zinc into leaf tissue adjacent to the application spots or into the leaves above or below the treated leaves. Given these problems with foliar zinc, Crowley et al (1996) suggest that fertilisation using soil or irrigation applied zinc sulphate may provide the most reliable method for correction of zinc deficiency in avocado on calcareous soils.
Whiley and Pegg (1990) report that foliar applications of zinc have been found to be highly ineffective in Queensland orchards.
Price (1990) reports that zinc can be absorbed through the leaves (from foliar sprays, e.g. zinc sulfate, zinc chelate) but that insufficient zinc can be absorbed in this manner to meet the plants requirements, especially in avocados. Since zinc is required at the growing points of new roots and shoots, it is essential that most zinc be taken up by the roots.
Foliar fungicide sprays
If leaf applied nutrient sprays in avocado give inconsistent or nil effects why do foliar sprays of phosphorous acid work for the control of root rot? The amount of phosphorous acid uptake required for root rot control is small but even so, several applications per year are required to be effective and the canopy must be dense and healthy. The phosphonate concentration required in the roots for effective root rot control is in the order of 30 mg/kg. To achieve this level either three to four sprays of 0.5% phosphorous acid per year are required at strategic times (Leonardi et al., 2000) or alternatively six or more sprays of 0.16% phosphorous acid per year must be applied. Another factor contributing to the effectiveness of leaf applied phosphorous acid is that, unlike many nutrients, it is extremely mobile in the plant.
Borys (1986) reports the dry matter distribution of roots to shoots in avocado seedlings average 26% and 74% respectively. Using these figures and some critical nutrient and fungicide levels in avocado we can get some perspective on the relative quantities required. In a tree consisting of say 100 kg of dry matter, about 26 kg would be in the roots and 74 kg in the shoots. This tree with a phosphonate root level of 30 mg/kg would contain a total of about 0.8 g phosphonate in the roots. With the optimal leaf levels of 50 mg/kg of boron and 2.5% of nitrogen, the tree would contain about 4 g and 1850 g of boron and nitrogen respectively in the canopy alone. It can be seen from these relative amounts that the fungicide required is substantially less than the nutrients.
Conclusion
Apart from well-timed boron applications at flowering in situations where leaf boron levels are deficient, there is no clear evidence to support the use of foliar nutrient sprays in avocado to correct nutrient deficiencies or to supply nutrients for growth. Occasionally a foliar nutrient spray may succeed in alleviating leaf deficiency symptoms, however this type of application will not provide the tree’s longer-term requirements for this nutrient which should be addressed through soil applications.
Acknowledgments
I would like to thank Drs Chris Searle and Tony Whiley and Mr Garry Fullelove of the Queensland Horticulture Institute for their assistance in compiling this article. The literature search was conducted using the AVOINFO avocado reference database.
Bibliography
Aziz, A.B.A., Desouki, I., El-Tanahy, M.M., Abou-Aziz, A.B. and Tanahy, M.M., El 1975. Effect of nitrogen fertilization on yield and fruit oil content of avocado trees. Scientia Horticulturae, 3 (1): 89-94.
Blanke, M.M. and Lovatt, C.J. 1993. Anatomy and transpiration of the avocado inflorescence. Annals of Botany, 71 (6): 543-547.
Borys, M.W. 1986. Root/shoot relation and some root characteristics in seedlings of avocado and Chinini. California Avocado Society Yearbook 70: 175-198.
Crowley, D.E., Smith, W., Faber, B. and Manthey, J.A. 1996. Zinc fertilization of avocado trees. HortScience 31 (2): 224-229.
Galindo-Tovar, G.E. 1983. Effects of urea spray concentration and surfactants on avocados. M.S. Thesis, University of California, Riverside, USA. September.
Gregoriou, C., Papademetriou, M. and Christofides, L. 1983. Use of chelates for correcting iron chlorosis in avocados growing in calcareous soil in Cyprus. California Avocado Society Yearbook 67: 115-122.
Jayanath, I. and Lovatt, C.J. 1995. Efficacy studies on prebloom canopy applications of boron and/or urea to 'Hass' avocados in California. World Avocado Congress III, Proceedings: 181-184.
Kadman, A., and Lahav, E. 1971-1972. Experiments with various treatments to cure chlorotic avocado trees. California Avocado Society Yearbook. 55:176-178.
Kadman, A. and Cohen, A. 1977. Experiments with zinc applications to avocado trees. California Avocado Society Yearbook, 61: 81-85.
Klein, I. & Zilkah, S. 1986. Urea retention and uptake by avocado and apple trees. Plant Nutr. 9:1415-1525.
Leonardi, J., Whiley, A.W., Langdon, P.W., Pegg, K.G. and Cheyne, J. 2000. Progress on the use of foliar applications of phosphonate for the control of phytophthora root rot in avocados.
Mans, C.C. 1996. Effect of foliar feeding of ‘Hass’ at various stages of flowering. South African Avocado Growers' Association Yearbook, 19: 31-32.
Nevin, J.M., Lovatt, C.J. and Embleton, T.W. 1990. Problems with urea-N foliar fertilization of avocado. Acta Horticulturae 275: 535-541. International Symposium on the Culture of Subtropical and Tropical Fruits and Crops. Vol. II. (J.C. Robinson, ed.), International Society for Horticultural Science. Wageningen, Netherlands.
Price, G. 1990. Thinking about zincing your trees? Talking Avocados, Third Edition, Aug/Sept, p.5.
Sing, J.L. and McNeil, R.J., 1992. The effectiveness of foliar potassium nitrate sprays on the 'Hass' avocado (Persea americana Mill.), World Avocado Congress II, Proceedings: "The Shape of Things to Come" (Lovatt, C.J. ed.) 1: 337-342.
Veldman, G. 1983. Calcium nitrate sprays on avocados at Westfalia Estate with the objective to reduce pulpspot. South African Avocado Growers' Association Yearbook, 6: 64-65.
Whiley, A.W., Chapman, K.R. and Saranah, J.B. 1988. Water loss by floral structures of avocado (Persea americana cv. Fuerte) during flowering. Australian Journal of Agricultural Research, 39 (3): 457-467.
Whiley, A.W., and Pegg, K.G.1990. Correction of micro-nutrient deficiencies and control of Phytophthora root rot in avocado. Talking Avocados, Second Edition, May/June, p. 11.
Whiley, A.W., Smith, T.E., Saranah, J.B. and Wolstenholme, B.N. 1996. Boron nutrition of avocados, Talking Avocados, 7 (2): 12-15.
Whiley, A.W., Hofman, P.J and Coates, L.M. 1997. From seed to tray - some field practices to improve avocado fruit quality. Proceedings of the Australian Avocado Growers' Federation and the New Zealand Avocado Growers' Association Conference '97, 'Searching for Quality'. Rotorua, New Zealand, pp. 83-97.
Zilkah, S., Klein, I., Feigenbaum, S. and Weinbaum, S.A. 1987. Translocation of foliar-applied urea 15N to reproductive and vegetative sinks of avocado and its effect on initial fruit set. J. Amer. Soc. Hort. Sci. 112:1061-1065.