Calag Archive
Calag Archive
Almond pruning wounds, bark abrasions susceptible to Ceratocystis
Publication Information
California Agriculture 50(3):29-33. https://doi.org/10.3733/ca.v050n03p29
Published May 01, 1996
Abstract
In the past, pruning wounds were not regarded as potential infection sites. However, in a 2-year study, pruning cuts inoculated immediately or at intervals up to 14 days were found to be susceptible to Ceratocystis canker from September through February. Broken, dead or living twigs became infected throughout the year when inoculated with the fungus.
Full text
Ceratocystis cankers enlarge slowly over several years, with the margins of active cankers outlined by amber-colored gum balls. Infected limbs frequently die, but the disease seldom destroys the tree.
Ceratocystis canker, also known as mallet wound canker, is a fungus disease of stone fruit trees most commonly found on almond and, occasionally, prune trees. Highly susceptible almond cultivars include ‘Mission,’ ‘Nonpareil’ and ‘Ne Plus Ultra.’ The cankers are sunken, dark and perennial. They enlarge slowly over several years, with the margins of active cankers outlined by amber-colored gum balls. Infected limbs frequently die, but the disease seldom destroys the tree.
Ceratocystis infections are associated with deep wounds that expose the cambium. Left, trees are often wounded during harvest by mechanical shakers. Below, ultimate canker size was affected by wound age, but not by time of year when infected. Fresh wounds were most at risk.
Infections are associated with deep wounds that often expose the cambium and are made during harvest by mechanical shakers or when knocking the branches with mallets to remove nuts. Improvements in the design and use of harvesting equipment have eliminated many of these wounds, thus dramatically reducing the incidence of this disease in California.
The pathogen, Ceratocystis fimbriata, is transmitted by several insect species that become contaminated with spores while visiting infected wounds. If attracted to fresh bark wounds, the insects then carry the spores to the new wounds where the fungus invades the cambium and surrounding vascular and cortical tissues. Although almond trees are susceptible any time of year, infection occurs most commonly in fall because of harvest-related injuries and insect vector activity. The fungus over-winters in old infections and with certain insects.
Ceratocystis infections also develop near pruning wounds or bark injuries unrelated to harvest. Cankers associated with pruning wounds are believed to be extensions of existing infections from the removed branch rather than new infections. Pruning wounds are not regarded as potential infection sites because they are of little interest to insects and because pruning usually occurs during winter when few vectors are present.
We recently isolated C. fimbriata from small cankers surrounding twigs and fruit spurs in several almond orchards. These cankers had marginal gumming, but were not associated with any obvious wounds as is typical of C. fimbriata infections. Several small cankers together can girdle and kill a branch.
The danger of infection by C. fimbriata as a consequence of harvest injuries is well recognized. However, injuries made during pruning operations, whether through pruning cuts or small inconspicuous wounds, have not been implicated as important infection sites. Here, we report on our research regarding (1) the susceptibility of pruning and small bark wounds; (2) effects of wound severity, age, and time-of-year on infection success; and (3) survival of the pathogen on bark surfaces.
The experiments
We conducted the study at the UC Kearney Agricultural Center in Fresno County. ‘Nonpareil’ almond trees were used for pruning wound experiments, while ‘Mission’ almond trees were hosts for the other experiments. Limbs selected for inoculation were 3-to-6-year-old secondary or tertiary smooth-barked scaffolds, 2 to 4 inches (5 to 10 cm) in diameter. Inoculum was produced from an isolate of C. fimbriata originally recovered from a canker surrounding a dead twig on a ‘Mission’ almond tree in a commercial orchard in Fresno County. Except where noted 0.007 fl oz (0.2 ml) of an aqueous suspension of 3 x 105 spores/fl oz (104 spores per ml) was applied to each inoculation site. Representative tissue samples from each experiment were cultured to reisolate C. fimbriata. The fungus was recovered from some but not all representative samples in the experiments.
Susceptibility of pruning wounds
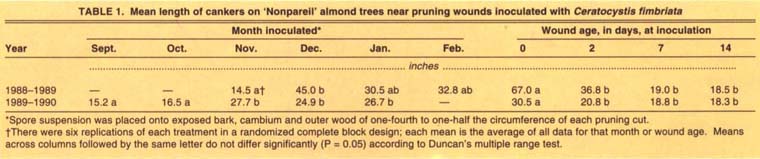
We tested the susceptibility of pruning wounds made monthly from November 1988 through February 1989, and from September 1989 through January 1990. Branches were removed with a chain saw, leaving a stub 2 to 3 feet (60 to 100 cm) long, 0, 2, 7, and 14 days before inoculation on the same date each month. In the 1988–89 test, approximately 0.008 fl oz (0.25 ml) of inoculum was applied to two opposite quadrants of the circumference of the exposed bark ring on each cut, with the noninoculated quadrants on each cut serving as controls. Only one quadrant of the circumference was similarly inoculated in the 1989–90 test. Controls were other noninoculated branches pruned on the same day. For the first experiment, canker length was recorded in September 1989 and for the second in August 1990. Each treatment was replicated six times using a randomized complete block design with adjacent individual trees as blocks. We calculated average canker lengths from all wound age inoculation data for each month.
Pruning wounds were susceptible to infection every month tested both years (table 1). In the first year, average canker length was significantly shorter in November than in the December inoculation, but lengths differed little among other months. In the second year, canker lengths were significantly shorter in pruning wounds inoculated in September and October than in later inoculations. Cankers at pruning cuts inoculated immediately were significantly longer than those at older wounds in both years.
Noninoculated quadrants died back 0.5 to 2.5 inches (1.0 to 6.0 cm) and 1.0 to 2.5 inches (2.5 to 6.0 cm) in the first and second experiments, respectively, which was significantly less (P = 0.05) than average canker extension for inoculated treatments (data not presented). This dieback apparently was a natural response to injury as no pathogens, including C. fimbriata, were isolated from these control treatments.
TABLE 1. Mean length of cankers on ‘Nonpareil’ almond trees near pruning wounds inoculated with Ceratocystis fimbriata
Creating small wounds
Small injuries were made by puncturing the bark to three depths, using a lancet with a bevel-tipped blade approximately 0.25 inches (4 mm) long and less than 0.1 inch (1 mm) in diameter. To determine the depth reached by the lancet, we estimated bark thickness by measuring four to six bark plugs taken with a cork borer from branches the same age and size as those used in the experiment. Because the bark was about 0.25 inches (3 to 4 mm) thick, full insertion of the lancet extended to the cambium or slightly beyond. A shallow wound that reached approximately midway between the surface and the cambium was standardized as follows: a bark plug was cut radically to half its depth, then the lancet blade was inserted through the slice. When pressed against the branch, the tip of the adjusted lancet pierced the bark without reaching the cambium. Scratches — superficial breaks of the outer bark — less than 0.1 inch (1 to 2 mm) long, were made with the lancet tip. Puncture wounds were located within 0.5-to-0.75-inch (1.5 to 2 cm) diameter circles. Open wounds that exposed the cambium were made by removing a 0.25-inch (5 mm) diameter bark plug with a cork borer. Inoculation sites were spaced 6 to 10 inches (15 to 25 cm) apart and on all sides of the branch, unless otherwise stated. Bark was not surface-sterilized before wounding, but the cork borer was decontaminated between cuts using alcohol dip and flaming. When not being used, lancets were stored in vials containing 70% ethyl alcohol. Wounds were not covered before or after inoculation unless otherwise stated.
Wound severity
Wound severity was varied in a standardized manner by altering the number and depth of punctures. In September 1988, punctures were kept at full depth, with the number of punctures (0, 2, 4, 6, or 8) varied in one experiment. In a separate experiment, the number of punctures was unchanged at eight for full-depth, half-depth, and scratch puncture wounds. All sites were inoculated immediately after wounding; injured, noninoculated sites served as controls. There were six replications (three per branch) of each treatment arranged in a randomized complete block design, with each experiment located on a different tree.
We evaluated the experiments in November 1988, using the presence of gum and internal necrosis as evidence of infection. Cankers formed at 83.3% and 67.7% (not significantly different) of full- and half-depth puncture wounds, respectively. The percentage of infected wounds increased significantly (r′ = .886) with the number of punctures per wound. Percent of infected wounds were 0.0, 50.0, 83.3, 100.0, and 100.0 for zero, two, four, six and eight full-depth punctures, respectively. Neither noninoculated nor scratch puncture wound sites were infected.
In the 1989 experiment, puncture number and depth were also varied. Two, four, six, and eight each of the full-depth, half-depth and scratch punctures were inoculated immediately after wounding. Controls were injured, noninoculated sites of the same wound depth and number of punctures. The experiment was initiated in September and December, 1989 and evaluated in August 1990. Treatments were replicated six times and arranged in a two-way factorial with split plot design.
Fig. 1. Effect of number and depth of puncture wounds in ‘Mission’ almond bark on susceptibility to infection by Ceratocystis fimbriata. Full-depth and half-depth punctures reached the cambium and cortex, respectively, scratch wounds were superficial breaks of the periderm. Wounds were inoculated immediately in September and December 1989; and evaluated August 1990. r′ = index for linear regression in an r (r = numbers of treatments) x 2 frequency table, P = 0.05 or less. NS = not significant.
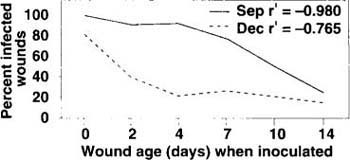
Fig. 2. Effect of wound age on susceptibility to infection of ‘Mission’ almond bark by Ceratocystis fimbriata. Bark plugs, 5 mm in diameter, removed at intervals (days) with a cork borer; insects excluded by covering wounds with Lumite saran between wounding and inoculation. Wounds inoculated on September 1988 and December 1989, evaluated on September 1989 and August 1990, respectively. r′ = index for linear regression in an r (r = number of treatments) × 2 frequency table, P = 0.05 or less.
The percent of infection did not differ significantly among sites wounded with two, four, six, or eight punctures (fig. 1A). However, significantly more cankers developed at full- and half-depth than at scratch puncture wounds (fig. 1B). No control wounds were infected. Mean canker length ranged from 2.0 to 3.2 inches (5.3 to 8.1 cm), with no significant differences in canker lengths among puncture numbers or depths.
Wound age
Open wounds that were 0, 2, 4, 7, 10 and 14 days old, made with a cork borer, were inoculated on the same day. Controls were similar wounds of each age treated with 0.007 fl oz (0.2 ml) of 0.4% water agar. All wounds were covered with Lumite Saran after wounding but before inoculation to exclude insects. The four single-tree replications of each treatment were arranged in a randomized complete block design. The experiment was conducted in September 1988 and December 1989, and evaluated the following summer of each year. Percent infection decreased with wound age, and wound susceptibility declined more rapidly in December than in September (fig. 2). All control wounds healed and were not infected.
Time of year
The susceptibility of small wounds at different times of the year was examined using five wounding techniques: (1) dead or (2) living twigs wounded by breaking them at the base without visibly tearing the bark or subtending branch, (3) eight full-depth punctures of the collar (the slightly enlarged roll of tissue found at the base of branches and twigs) of dead twigs, (4) eight full-depth bark punctures, and (5) bark plugs removed with a cork borer.
Wounds were inoculated immediately; controls were similar uninjured, inoculated sites. The experiment was performed in September and November of 1988, and January, March, May and July of 1989. There were 12 replications of each treatment each month, with each replication assigned to one branch, using four branches on each of three trees. The 12 replications were divided into two groups of six (two limbs from each tree), with groups organized in randomized complete block designs. One group from each inoculation month was sacrificed in October 1989 for measurement of canker length and reisolation of the fungus. The remaining groups were kept for further observation of symptoms and evaluated in August 1990.
Cankers formed every month at all inoculated collar and bark puncture and cork borer wounds, and infections were more frequent near broken living than broken dead twigs (table 2). Bark wounds were infected in each of the 6 months tested, and there were no significant differences in percent infection among months (data not presented). Control sites were not infected except for one uninjured dead twig inoculated in May. Mean canker length (derived from data combined for all months) at cork borer injuries was significantly longer, (7.0 inches, 17.6 cm) than canker length at collar puncture wounds near dead (3.8 inches, 9.7 cm) or living twigs (2.5 inches, 6.5 cm). Treatment groups maintained for 2 years to observe symptoms showed similar infection percentages. The cankers became more sunken, continued to gum along the margins and resembled cankers originally observed in commercial orchards. Three branches died.
Survival on bark surface
Fungus survival on bark surface was tested using a collection of spores and mycelial fragments gathered by drawing the edge of a small spatula across the surface of 14-to-21-day-old cultures. Inoculation sites were identified by drawing 0.75 inch-diameter (2 cm) circles spaced 10 inches (25 cm) apart along one side of a branch. A companion circle, to be used as a noninoculated control, was drawn for each inoculation circle on the opposite side of the branch. Inoculum was smeared on all inoculation circles on November 9, 1989, after which eight full-depth punctures were made in the inoculation and companion control circles at the following intervals, in days: 0, 2, 7, 14 (November), 30 (December), 61 (January), 103 (February), 141 (March), 165 (April), 190 (May), and 222 (June). Because rain may wash spores into wounds, the inoculated and companion control sites were wounded during rains on January 13 and 30. Treatments were replicated four times, with each replication housed on one of four trees, and arranged in a randomized complete block design.
Infections occurred at sites inoculated when wounded immediately or 2, 7, or 14 days later. All sites except one in the 14-day-old treatment were infected, and a canker developed at one site wounded January 30 during rain. No cankers were observed at any other inoculated or noninoculated locations.
Fresh wounds most at risk
Pruning wounds were susceptible to infection by C. fimbriata during fall and winter, and remained susceptible for at least 14 days. Fresh wounds were most at risk and ultimate canker size was affected by wound age but not by time of year when infected. Wounds became less susceptible with time, and wound severity — represented in these tests by the number or depth of punctures — was less important than age in determining the success of infection.
Using a lancet, puncture wounds were made to the trees. The number or depth of punctures was less important than age of the wound in determining susceptibility to infection.
Injuries made during pruning are good candidates for infection by C. fimbriata. Pruning cuts, along with bark abrasions or small breaks near twigs made when pruned branches are pulled from the canopy, provide many potential infection sites. The broken twig, puncture and scratch wounds used here were designed to mimic small wounds that may be created during pruning. These were susceptible to infection throughout the year, and the associated cankers were similar to those found on almond tree branches in commercial orchards.
Pruning usually occurs during late fall and winter when few insects are present or active. Although small wounds are adequate courts for infection, their attractiveness to insect vectors — which is not high — is equally important for natural spread of the fungus. Insects have been observed visiting large bark wounds shortly after injury, and remaining to infest the wound. Insects may also be attracted to small, inconspicuous wounds, but this behavior is not as obvious. They may further disseminate spores by inadvertently scattering them along the bark during general activity.
According to our evidence, C. fimbriata does not survive easily on bark for long periods when unprotected, but further investigation may prove otherwise. One wound became infected when injury was made during rain, which indicates that some inoculum survived and rain may play an important role in infecting small wounds. Spores on bark surfaces may be moved about by rain and washed into wounds, but this has not been demonstrated. Growers have reported greater incidence of cankers at pruning wounds and along branches in trees pruned just before rain. The minimal activity of vectors in winter when most pruning occurs and the random chance of possible rain dispersal may, in part, explain why infection of pruning or small wounds is not more prevalent in nature.
Unfortunately, our research did not identify a time of year when pruning can be done without risk of infection. At present, the best recommendation to growers is to avoid injuring bark with harvesting equipment, thus preventing establishment of the fungus in the orchard, and to prune when least likely to be followed by rain.