Calag Archive
Calag Archive
New technique predicts gray mold in stored kiwifruit
Publication Information
California Agriculture 50(3):34-40. https://doi.org/10.3733/ca.v050n03p34
Published May 01, 1996
Abstract
Sampling fruit from vineyards 4 months after fruit set and recording the incidence of Botrytis colonization in sepals or stem ends was used as a field-monitoring method to predict the incidence of kiwifruit Botrytis gray mold after 3 or 5 months in cold storage. Spraying the fungicide vinclozolin one or two weeks before harvest significantly reduced postharvest gray mold after 5 months storage only in vineyards with more than 6% gray mold. Preharvest sprays were not needed when expected gray mold was below 6%. The method reported here can be used successfully by growers to make decisions about preharvest sprays, sorting, repacking and when to market and ship fruit.
Full text
Kiwifruit are sorted for defects, primarily gray mold. Fruit loss and the costs for re-sorting and repacking can add considerably to the final price of the product.
Approximately 6,300 acres of kiwifruit (Actinidia deliciosa) are grown in California each year, producing 10 million to 11 million boxes of fruit valued at $100 million to $110 million. Although kiwifruit initially was thought to be a “disease-free” crop in California, increasing acreage and expanding exports over the last 10 years have increased the quantity of fruit in cold storage. Postharvest rotting due to Botrytis gray mold has become a potentially serious problem.
This postharvest decay is a direct result of Botrytis cinerea infections that occur in the field but remain latent in the senescent floral parts (sepals and stamens), stem-end scars, or small wounds inflicted during fruit harvest. During long-term cold storage, the kiwifruit become physiologically susceptible to the pathogen, which usually mvades the fruit tissues from the stem end.
Research in 1983 (California Agriculture January-February 1983) showed that bloom and preharvest spraying with vinclozolin (Ronilan 50WP) — which is registered for control of Botrytis rot in kiwifruit — significantly decreased the incidence of postharvest gray mold. Best control of Botrytis rot was achieved with four fungicide applications (two at bloom and two at preharvest), but this may not be economical when the cost of the fungicide applications exceeds loss from rot. In addition, four applications per year of vinclozolin increase the risk of developing resistant strains of the pathogen. As a result of these problems, along with environmental issues and public concern regarding pesticide usage, efforts are being made to discover alternatives or to reduce usage.
Fig. 1. Sepals and stem ends were separated for isolation and plating to determine colonization by Botrytis cinerea.
Decay during transit
Controlled atmosphere (CA) technology has extended the storage life of kiwifruit from a few weeks to 5 or 6 months. Although near-optimum conditions can be achieved in commercial storage, conditions during transport to distant export markets are often less than optimum. With transit times of up to 30 days, there is potential for considerable gray mold decay and loss of fruit quality during shipment from California to European markets. Fruit loss and the costs for re-sorting and repacking can add considerably to the final price of the product. Unfortunately, the incidence of Botrytis gray mold is unpredictable since it is highly variable from year to year and from vineyard to vineyard. Up to 20% incidence of Botrytis stem-end rot has been recorded in California, with a range of 32% to more than 50% for kiwifruit from Italy and New Zealand. In California kiwifruits, decay is limited to fruit in storage or in marketing channels, with environmental conditions and field location influencing the severity of damage. In our trials, vineyards that had the highest levels of infection in floral parts also had the highest fruit rot after 6 1/2 months in storage.
Presently there is no reliable method for predicting the development of gray mold during cold storage so some growers routinely apply one preharvest spray of vinclozolin regardless of the expected levels of disease. Shippers also lack reliable criteria for determining which fruit to sell first and which to keep for longer storage. In the past, shippers generally have relied on historical incidences of Botrytis gray mold in a field. However, if the incidence of kiwifruit sepals or stem ends colonized by B. cinerea is a correct predictor of decay in storage, it is possible to do one or more things: (1) send first to market those fruit expected to have higher incidence of gray mold and hold in storage those expected to have lower incidence of gray mold; (2) only spray fields expected to produce fruit with high potential for disease; (3) reduce the number of fungicide applications to fields with medium disease potential; or (4) avoid spraying fields that have low or nil disease potential.
The purposes of this study were to determine the relationship between colonization of kiwifruit sepals and stem ends by B. cinerea and the incidence of gray mold in cold storage, and to determine when spraying vinclozolin in kiwifruit vineyards is justified.
Field sampling (1993 and 1994)
In 1993 and 1994, three samples of 20 fruit were collected arbitrarily 2, 3 and 4 months after fruit set, and 1 week before and during commercial harvest. In 1994, fruit samples were also collected 1 month after fruit set. Vineyards used in 1993 included three each in Butte County (Sacramento Valley), Kern County (San Joaquin Valley), and San Luis Obispo County (coastal area). Vineyards used in 1994 included two in Butte County, three in Kern County, one in Fresno County, and three near the coast, in San Luis Obispo and Santa Cruz counties. All vines were trained using a standard T-bar system and irrigated by either drip or fan-jet irrigation. Fruits were collected with stems intact and placed in plastic holders in one-layer boxes to prevent discharge of sepals.
We cut fruit tissue bearing sepals and stem ends with a sharp sterile knife, then separated the sepals from the stem ends by hand (fig. 1). Sepals were surface-disinfected in a 0.5% sodium hypochlorite solution for 1 to 2 minutes, washed in sterile distilled water and plated in petri dishes containing acidified (pH 3.5) potato-dextrose agar (APDA). (This method also had been used successfully in preliminary experiments in 1992.) Five or six sepals and one stem end from the last three sampling dates were placed in petri dishes and the dishes incubated at 44°F (7°C) for 6 days. We marked colonies of B. cinerea growing from plated sepals or stem ends, and incubated the dishes at 73°F (23°C) for 3 more days, then counted growing colonies of B. cinerea. Incubating the dishes at the lower temperature was necessary to reduce the incidence of contamination by Rhizopus spp., which occasionally grew in the samples. Using a salt shaker to sprinkle the plates with dicloran (Botran 75 WP) prevented further Rhizopus growth.
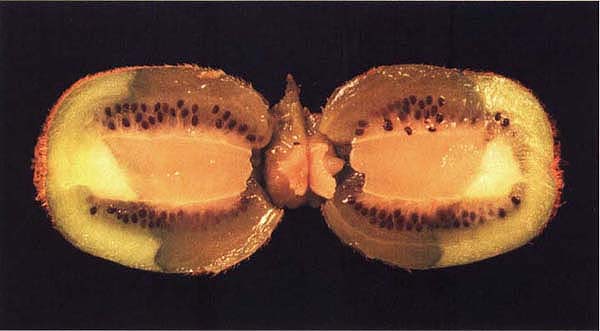
This cross-sectioned fruit with stem-end rot shows water-soaked tissues, a sign of decay by Botrytis cinerea.
Fruit harvest, storage
At commercial harvest in both 1993 and 1994, 38 one-layer boxes with 33 fruit (6.5° Brix, 6.5% soluble solids concentration) per layer per vineyard were harvested and stored in a commercial CA cold storage facility (31°F [-0.5° C] and 8 parts per billion [ppb] ethylene) in Kingsburg, Fresno County. One Butte County vineyard was harvested by the grower before the 1994 sampling; therefore, fruit from that vineyard was not sampled. The incidence of fruit infected with gray mold during storage was recorded after 3 and 5 months. To prevent secondary spread of the disease in storage, all Botrytis-infected fruit was discarded after the first recording.
Levels of colonization of both sepals and stem ends by B. cinerea 4 months after fruit set were classified in three categories: low (0–15%), medium (16–50%), and high (more than 50%). Since postharvest decay of kiwifruit after 3-month storage did not exceed 5%, we established two different sets of levels of decay. The 3-month CA storage included low (less than 1%), moderate (1–3%), and high (3–5%). For the 5-month CA storage, levels were low (less than 2%), moderate (2–6%), and high (more than 6%).
Fungicide sprays (1991–1994)
In 1991, five vines in a commercial vineyard in San Luis Obispo County were sprayed with vinclozolin (2 lb/100 gal of water) one week before commercial harvest on November 14, with five vines serving as unsprayed controls. In 1992, 10 vines in the same county were sprayed with vinclozolin, using the same 2 lb/100 gal of water ratio, either during full bloom May 25 or one week preharvest on October 26, or in two sprays, at full bloom and one week preharvest, using a knapsack-type power duster and mist blower. In a Kern County vineyard, only the preharvest spray was applied on September 28, 1992, one week before commercial harvest. In each vineyard, 10 unsprayed vines served as controls. Six boxes of 33 fruits (6.5° Brix) per replicated vine were harvested on November 2 in San Luis Obispo County, and five boxes of 39 fruits per replication from the Kern County plot were harvested on October 5. All fruit were CA-stored as described above.
In 1993, two trials arranged in a randomized complete-block design were set in commercial vineyards in Butte and Kern counties. In both cases, 10 vines were sprayed once, either at full bloom May 5 or one week before harvest on October 12, or twice, at full bloom and again one week before harvest. In each trial, 10 unsprayed vines served as controls. Two boxes each of 33 fruit (6.5° Brix) per replicated vine were harvested one day before commercial harvest and stored at 31°F (-0.5°C) and 8 ppb ethylene for 5 months.
In 1994, five vines in each of the four commercial kiwifruit vineyards were sprayed with vinclozolin either one or two times, 1 and 2 weeks before harvest. The dates of fungicide application varied for all four vineyards, depending on different maturation dates for fruit. Each trial was arranged in a randomized complete-block design, with one vineyard in San Luis Obispo (expected high incidence of gray mold), two in Kern County (expected moderate to low incidence), and one in Butte County (expected low incidence). Vinclozolin was applied on October 25 or October 31, or both dates, in the San Luis Obispo vineyard; on September 27 or October 3, or both dates, in both vineyards in Kern County; and on September 28 or October 7, or both dates, in Butte County. The rate and method of application were the same as previous years. At commercial harvest time, five boxes of 39 fruit (6.5° Brix) each were harvested from each replicated vine, packed commercially, and stored in commercial CA storage as described above. Botrytis gray mold was recorded after 3 and 5 months storage for each year's experiment. To prevent secondary spread of decay, we discarded all decayed fruit recorded after 3-month storage.
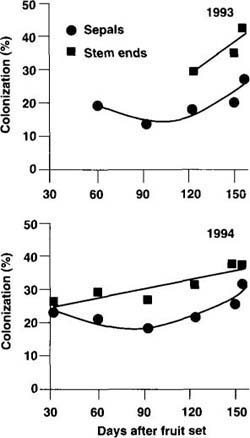
Fig. 2. Progress of colonization by Botrytis cinerea sepals and stem ends of kiwifruit in nine vineyards each in 1993 and 1994 in California. Data points represent the means of nine vineyards per year with > 300 sepals and 60 receptacles per vineyard in each sampling date.
Statistical analyses
We combined data from two recordings of B. cinerea (after 6 days at 44° F [7°C] and 3 days at 73°F [23°C]). The percent of colonization of sepals and stem ends by B. cinerea in both 1993 and 1994, and the incidence of gray mold after 3- and 5-month cold storage, were analyzed with regression analysis using the REG procedure of the SAS statistical methods. Results were similar for sepal or stem-end colonization by B. cinerea and Botrytis gray mold developed in CA storage; therefore, 1993 and 1994 results were averaged for the 4-month sampling and presented as regressions.
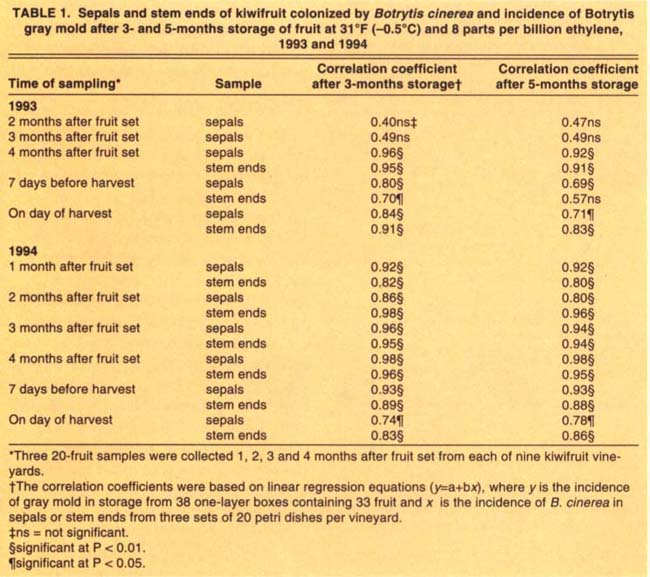
TABLE 1. Sepals and stem ends of kiwifruit colonized by Botrytis cinerea and incidence of Botrytis gray mold after 3- and 5-months storage of fruit at 31°F (-0.5°C) and 8 parts per billion ethylene, 1993 and 1994
Botrytis dips after fruit set
Because similar trends were noted in the levels of colonization of sepals and stem ends by B. cinerea in the various vineyards, the data were averaged over all vineyards. In both years of testing, sepal colonization decreased 3 months after fruit set, but increased afterwards until harvest (fig. 2). Colonization of stem ends by B. cinerea increased linearly with time as fruit approached maturity, and the data in 1994 were best described with a positive linear equation (fig. 2).
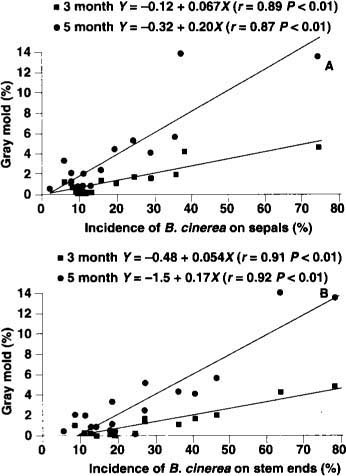
With the exception of the two first samplings in 1993, there was significant positive correlation between the levels of B. cinerea colonization of sepals and stem ends and the incidence of Botrytis mold decay on kiwifruit after 3 and 5 months cold storage (table 1). For both years, the strongest relationship was obtained in fruit sampled 4 months after fruit set and after 3 months in cold storage (table 1). Some secondary spread of the fungus may explain the lower correlation coefficients for the 5-month storage of fruit (fig. 3).
On the other hand, the correlation coefficients for colonization of sepals and stem ends sampled at harvest were lower than those at other sampling times, suggesting that sampling at commercial harvest time will under-estimate the incidence of gray mold in storage.
For both years of testing, the three levels of colonization of sepals or stem ends by B. cinerea 4 months after fruit set corresponded to the majority of vineyards having a low, moderate or high level of gray mold decay at the end of the CA storage period. For example, after 3-month CA storage, the levels of sepal and stem-end colonization by B. cinerea predicted correctly the postharvest gray mold decay in 8 out of 9 (89%) and 7 out of 9 (78%) vineyards in 1993, and 7 out of 8 (88%), and 6 out of 8 (75%) vineyards in 1994, respectively (table 2). After 5-month CA storage, the same levels of sepal and stem end colonization predicted gray mold decay in 100% and 78% of vineyards in 1993, and 75% and 63%, of vineyards in 1994, respectively (table 3). These results suggest that both sepal and stem end colonization by B. cinerea 4 months after fruit set are reliable methods for predicting the incidence of gray mold decay after 3- or 5-month CA storage of kiwifruit. For both periods, sepal colonization by B. cinerea more accurately predicted incidence of gray mold.
Fig. 3. Postharvest Botrytis gray mold of kiwifruit kept at 31°F (-0.5°C) and 8 parts per billion ethylene for 3 months (square points) and 5 months (circle points). X axis shows incidence of Botrytis cinerea isolated from sepals (A) and stem ends (B) from fruit sampled 4 months after fruit set. Points are averages from nine vineyards in 1993 and eight in 1994.
TABLE 2. Relationship of levels of sepal and stem-end colonization by Botrytis cinerea in kiwifruit samples collected 4 months after fruit set and number of vineyards with low (<1%), moderate (1–3%), and high (> 3%) levels of Botrytis gray mold decay after 3 months in controlled atmosphere storage (31°F [-0.5°C] and 8 parts per billion ethylene), 1993 and 1994
TABLE 3. Relationship of levels of sepal and stem end colonization by Botrytis cinerea in kiwifruit samples collected 4 months after fruit set and number of vineyards with low (<2%), moderate (2–6%), and high (>6%) levels of Botrytis gray mold decay after 5 months in controlled atmosphere storage (31 °F [-0.5°C] and 8 parts per billion ethylene), 1993 and 1994
TABLE 4. Effect of one or two vinclozolin sprays in the field to reduce Botrytis gray mold of kiwifruit after 3-months cold storage (31°F [-0.5°C] and 8 parts per billion ethylene), 1991–1993
Effects of sprays (1991–1994)
In 1991, a preharvest spray of vinclozolin reduced the incidence of gray mold (P < 0.05) in the San Luis Obispo County vineyard (table 4). In 1992, spraying with vinclozolin 7 days before harvest did not significantly reduce gray mold in the Kern County vineyard. In the San Luis Obispo vineyard none of the sprays applied at bloom, and / or preharvest in 1992 significantly reduced the incidence of gray mold after 3 months of fruit storage (table 4). In the 1993 experiments, the level of Botrytis gray mold on fruit from untreated vines in the Kern County vineyard was very low (3.3%) and none of the vinclozolin sprays significantly reduced the incidence of gray mold after 3-month CA storage of fruit (table 4).
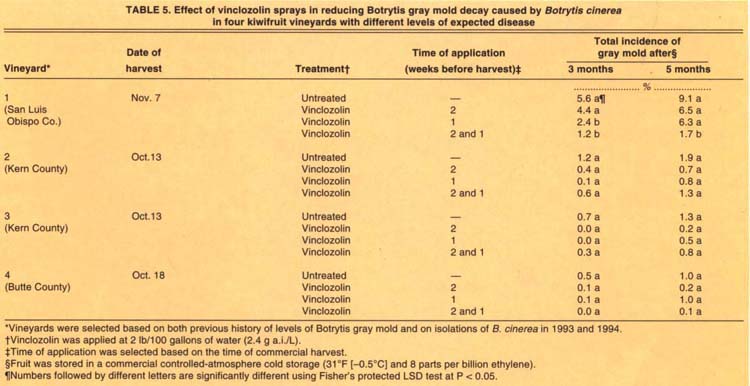
In 1994, only the sprays that included vinclozolin applied 1 week before harvest significantly reduced the incidence of Botrytis gray mold after CA storage, and then only in the San Luis Obispo plot where fruit from the unsprayed vines developed 5.6% and 9.1% incidence of gray mold after 3- and 5-month CA storage, respectively (table 5). Vinclozolin applied 2 weeks before harvest did not reduce disease. The levels of disease in fruit harvested from the other three plots ranged from 0.5 to 1.2%; fungicide applications resulted in no significant reductions of postharvest disease incidence (table 5).
Predicting gray mold
It is possible to use the incidence of colonization of sepals and stem ends by B. cinerea of immature fruit as a criterion for predicting the levels of postharvest gray mold decay of mature kiwifruit in commercial CA storage. In a preliminary study (1990 to 1991), we found that vineyards with fruit having low levels of sepal colonization by B. cinerea also showed low incidence of postharvest gray mold in cold storage. In 1992, a preliminary study showed a linear relationship between sepal infestation by B. cinerea and incidence of gray mold after 5-month CA storage of fruit. These initial observations prompted the present study.
To implement this new Botrytis monitoring system, growers can collect fruit samples (three samples of 20 fruit throughout the vineyard) 4 months after fruit set and submit them to a laboratory to determine the extent of colonization. By using averages from our 1993 and 1994 data (fig. 3), one can easily calculate the level of gray mold expected to develop after 3 or 5 months of storage. This prediction can also help growers decide whether preharvest vinclozolin applications are necessary.
The higher levels of stem-end colonization by B. cinerea than those of sepals (fig. 2) can be attributed to the fact that colonization of stem ends represents a far more advanced infection capable of progressing and invading fruit tissues during storage. In contrast, infected sepals can break off during packing before the fungus has invaded the stem end. But even with this deficiency, plating more than 300 sepals (three 20-fruit samples with 5 or 6 sepals per fruit) resulted in a greater success in predicting postharvest decay (75 to 100% vineyards) than stem ends (63 to 78% vineyards). Plating stem ends may provide an easier way to forecast storage rot (for three 20-fruit samples, it is necessary to plate only 60 stem ends), but it seems to be a less successful method for predicting Botrytis gray mold in storage. It takes only 6 days for initial results on colonization of sepals or stem ends in a diagnostic test, and 8 to 9 days for final results.
TABLE 5. Effect of vinclozolin sprays in reducing Botrytis gray mold decay caused by Botrytis cinerea in four kiwifruit vineyards with different levels of expected disease
By using the forecasting method described here, packing house operators and shippers can arrange to sell fruit lots with expected high incidence of sepal or stem end infection first and hold fruit with low incidence for longer storage. Alternately, if a diagnostic test conducted 4 months after fruit set shows a very low colonization of sepals by B. cinerea, re-sorting, repacking, and preharvest fungicides may not be necessary. If this approach is tested and proves successful in other countries where preharvest fungicide treatments with vinclozolin are used (i.e., New Zealand, Chile, Italy, Spain, Greece), it could eliminate treatment for vineyards showing low (0 to 15%) sepal or stem-end incidence of B. cinerea colonization.
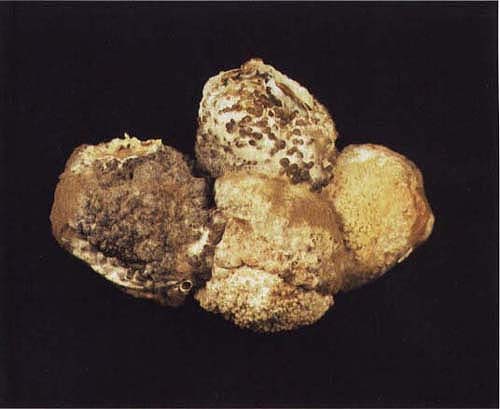
A cluster of fruit with gray mold. Note white (immature) and black (mature) sclerotia structures of the pathogen Botrytis cinerea.
Although selective and semi-selective media have been developed for isolating B. cinerea, the medium used in this study was a simple recipe (39 g of potato-dextrose agar per liter of medium acidified after autoclaving with lactic acid to pH 3.5), and the technique described here can easily be used by private consultants to predict Botrytis gray mold of kiwifruit in storage. Because of the simplicity and the low cost of the method developed in this study, growers can implement it immediately.
Most of the fruit in this “kiwifruit cemetery" was discarded because it had gray mold decay caused by Botrytis cinerea. The incidence of B. cinerea in immature fruit can be used to predict the levels of postharvest gray mold decay of mature kiwifruit in commercial, controlled-atmosphere storage.
Furthermore, we found fungicide application to be effective only when disease levels of unsprayed controls were higher than 6% (tables 4 and 5). Our studies also showed that only one spray one week before harvest significantly reduced disease. When disease levels were lower than 3%, spraying was not necessary. Thus, using the 4-month sampling to predict storage decay can be very useful in making decisions for two, one, or no preharvest sprays.
In summary, monitoring incidence of B. cinerea colonization of sepals and stem ends to predict Botrytis gray mold in stored kiwifruit is simple, inexpensive and quick. It provides growers with useful and practical criteria for preharvest spray decisions and informs packing-house operators and shippers to make better marketing and shipping decisions.



![Relationship of levels of sepal and stem-end colonization by Botrytis cinerea in kiwifruit samples collected 4 months after fruit set and number of vineyards with low (<1%), moderate (1–3%), and high (> 3%) levels of Botrytis gray mold decay after 3 months in controlled atmosphere storage (31°F [-0.5°C] and 8 parts per billion ethylene), 1993 and 1994](http://calag.ucanr.edu/archive/?file=tab5003p38a.jpg)
![Relationship of levels of sepal and stem end colonization by Botrytis cinerea in kiwifruit samples collected 4 months after fruit set and number of vineyards with low (<2%), moderate (2–6%), and high (>6%) levels of Botrytis gray mold decay after 5 months in controlled atmosphere storage (31 °F [-0.5°C] and 8 parts per billion ethylene), 1993 and 1994](http://calag.ucanr.edu/archive/?file=tab5003p38b.jpg)
![Effect of one or two vinclozolin sprays in the field to reduce Botrytis gray mold of kiwifruit after 3-months cold storage (31°F [-0.5°C] and 8 parts per billion ethylene), 1991–1993](http://calag.ucanr.edu/archive/?file=tab5003p38c.jpg)