- Author: Steven T. Koike
- Author: Mark Bolda
Beginning at least as early as 2005 and continuing through 2013, collapsing strawberry plants from various parts of California have been associated with the soilborne fungus Macrophomina phaseolina. The disease, called charcoal rot, appears to be the most important current concern for the industry due to its steady increase over this period of time. Each year finds additional new fields infested, and the disease has now been found in all of the major strawberry producing counties in the state. In 2005-2006, charcoal rot was restricted to southern California in Orange and Ventura counties. Most recently this disease has been confirmed in Santa Barbara, Monterey, Santa Cruz, and Santa Clara counties. The spread of Macrophomina to new fields and counties portends that charcoal rot may be a long term threat to the industry which at present does not have satisfactory plant resistance with which to combat the pathogen.
Symptoms of Macrophomina infection in strawberry consist of wilting of foliage, plant stunting, and drying and death of older leaves, with the central youngest leaves often remaining green and alive. Plants can eventually collapse and die (Figure 1). When plant crowns are cut open, internal vascular and cortex tissues are dark to orange brown (Figure 2). Disease is often most severe if the infected plant is subject to stresses such as weather extremes, water stress (shortage of water), poor soil conditions, or heavy fruit loads. In locations where the disease has occurred for more than one season, the patches can be quite large and appear to have spread from the initial problem area (Figure 3). Such patterns are consistent with the spread of a soilborne pathogen. It is noteworthy that in these cases we have never isolated other important, well known pathogens such as Colletotrichum, Phytophthora, or Verticillium. However, it is important to note that another recently described disease, Fusarium wilt, is also occurring in the same regions; symptoms of Fusarium wilt are identical to those caused by charcoal rot.
Macrophomina produces numerous tiny, black, irregularly shaped microsclerotia (Figure 4). These microsclerotia are survival structures that allow the fungus to persist for extended periods in the soil. The fungus is spread within and between fields mostly by the transport of contaminated soil during soil tillage and preparation operations. Spread of Macrophomina in strawberry fields deals with the same issue of field sanitation that concerns growers of many other commodities. Verticillium wilt (lettuce, strawberry, pepper), clubroot (broccoli, cauliflower), Fusarium wilt (lettuce), Fusarium yellows (celery), and lettuce dieback disease (lettuce) are all problems caused by soilborne pathogens that are spread in infested soil.
Current management strategies involve the following: (1) Crop rotation. Do not plant strawberry in fields having a known history of the problem and avoid back-to-back strawberry plantings in infested locations. (2) Pre-plant fumigation. This remains a useful tool for managing Macrophomina and the other soilborne pests, even though bed-applied fumigants may not provide complete control. (3) Avoid stressing the plants. Stress will hasten the development and increase the severity of symptoms, so use appropriate growing and irrigation practices to reduce stress. Note, however, that even in the absence of stress, infected plants will eventually develop the disease. (4) Sanitation. Growers with Macrophomina infested fields need to be concerned with limiting the spread of the fungus from infested to clean fields.
Figure 1. Charcoal rot results in the collapse and death of strawberry plants.
Figure 2. Internal crown tissue of strawberry infected with Macrophomina will show a dark to orange brown discoloration.
Figure 3. Charcoal rot can affect large portions of a field and cause significant dieback.
Figure 4. Tiny, black microsclerotia enable the Macrophomina pathogen to survive in the soil.
- Author: Richard Smith
- Author: Steven T. Koike
In the last year we have been called out to look at lettuce fields showing uneven growth. The fields looked like they were not growing normally due to a cultural practice. The lettuce plants in the field vary in size with normal plants interspersed with stunted plants (Photos 1 and 2). The patterns in the field do not fit with sprinkler patterns or mechanical issues because affected plants are more randomly dispersed. The pattern may fit with soil insect issues, but no soil insects were present in the affected fields. Sometimes stunted plants were more prevalent in areas that might have poor drainage. The randomness of affected plants is similar to some of the nitrogen toxicity issues that we have detected in some fields (http://ucanr.org/blogs/blogcore/postdetail.cfm?postnum=4931). In some cases the taproot of the plant is missing (a typical symptom of nitrogen toxicity), but taproots are not consistently missing and therefore the symptoms do not fit nitrogen toxicity. Upon close examination of the root system, small necrotic sections of the root are present and we were able to confirm that the lettuce was affected by black root rot disease.
Root symptoms of black root rot of lettuce consist of numerous dark brown to black bands and lesions on small feeder and larger secondary roots (Photos 3 and 4). Internal vascular discoloration (as seen with Verticillium wilt) is absent. If severe infections occur early in the crop cycle, the lettuce seedlings will have damaged taproots and experience delayed growth, resulting in stunted, small plants that otherwise may look healthy. The stunting is usually most evident from rosette stage through crop maturity. In advanced cases, the lower leaves of the plant may turn yellow or even brown. In the Salinas Valley, black root rot has been seen to cause stunting and resulting crop loss on both iceberg and romaine cultivars. Black root rot of lettuce has been known for many years in Australia and other regions. The disease was first documented on lettuce in California in 2005. Our diagnostic lab has periodically received black root rot cases since then.
Black root rot is caused by the soilborne fungus Thielaviopsis basicola. The fungus makes two types of spores, with one type being a thick walled spore (Photo 5) that resists weathering and enables the fungus to survive for long periods in the soil. This pathogen is reported to have a broad host range that includes the following vegetable crops: bean, beet, carrot, garlic, lettuce, onion, pea, pepper, tomato. In coastal California we have not found black root rot on most of these crops; black root rot disease has been confirmed only for lettuce and tomato.
Differences in strains or isolates of T. basicola may exist. For example, researchers found that when inoculated onto lettuce and bean, T. basicola isolates from lettuce were very aggressive and caused significant damage. However, the same lettuce isolates caused very little damage to other known hosts. Such research indicates that T. basicola may consist of isolates having some host preferences.
Industry-wide, black root rot of lettuce is a very minor problem that apparently only affects a few fields. However, growers and PCAs should be aware of this disease and notify Farm Advisors if black root rot becomes more common. For other crops, black root rot is managed by a combination of resistant cultivars, crop rotation to non-hosts, sanitation practices so as to not spread contaminated soil/mud to uninfested fields, and application of fungicides to seedlings.

Photo 1. Typical pattern of Thielaviopsis basicola infection in lettuce.

Photo 2. Stunted lettuce affected with black root rot (on left)

Photo 3: Diseased roots of lettuce affected by black root rot.

Photo 4. Close-up of black root rot lesion on fine feeder roots of lettuce.
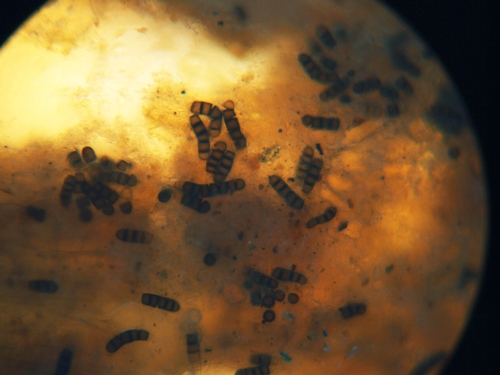
Photo 5: Dark conidia of Thielaviopsis basicola forming on lettuce root.
- Author: Steven T. Koike
Weather and lettuce diseases. Late spring rains, cold temperatures, and high humidity are making it possible for two important foliar diseases of lettuce to show up in California and cause damage in 2012. Both bacterial leaf spot and anthracnose have been observed in numerous fields throughout the central coast. If rains continue or if the crop is irrigated with sprinklers, both diseases can result in significant damage to lettuce leaves and resulting loss of yields due to poor quality of the harvested heads. Experienced field personnel will likely recognize these two problems without difficulty; however, we recommend that if there is any question, laboratory tests be conducted to confirm diagnosis.
Bacterial leaf spot. Initial symptoms are small (1/8 to 1/4 inch), water-soaked spots that occur on the older, outer leaves of the plant. Lesions are typically angular in shape and quickly turn black (photo 1)—this is the diagnostic feature of this disease and readily separates this from anthracnose (which causes white to light pink lesions). If disease is severe, numerous lesions may coalesce, resulting in the collapse of the leaf. Older lesions dry up and become papery in texture, but retain the black color. Lesions rarely occur on newly developing leaves.
Bacterial leaf spot is caused by bacterium Xanthomonas campestris pv. vitians. The pathogen is highly dependent on wet, cool conditions for infection and disease development. Splashing water from overhead irrigation and rain disperses the pathogen in the field and enables the pathogen to infect significant numbers of plants. The pathogen can be seedborne and is introduced into the field via contaminated seed. In addition, the bacterium can survive for up to five months in the soil. Therefore, infected lettuce crops, once disked into the soil, can supply bacterial inoculum that can infect a subsequent lettuce planting.
Spray applications are not effective at managing bacterial leaf spot of lettuce. The disease is managed by using uninfested seed, irrigating the crop via furrow or drip, and avoiding back-to-back lettuce rotations if the first crop was diseased.
Anthracnose. Early symptoms are small (1/8 to 1/4 inch), water-soaked spots occurring on outer leaves. Spots enlarge, turn yellow then tan, and are usually angular in shape. Under cool, rainy conditions, white to pink spore masses of the fungus will be visible in the centers of the tan colored lesions (photo 2)—this is the diagnostic feature of this disease and readily separates this from bacterial leaf spot (which causes black lesions). If disease is severe, the lesions will coalesce and cause significant dieback of the leaf and in some cases will result in stunting of the plant. As spots age, the affected tissue will dry up and become papery in texture. Eventually the centers of these spots can fall out, resulting in a shot hole appearance. Anthracnose lesions are often clustered along the midribs of lower leaves.
Anthracnose is caused by the fungus Microdochium panattonianum. This fungus infects only lettuce and does not cause disease on any other crop. The fungus can survive for up to four years as microsclerotia in soil. The anthracnose pathogen requires cool, wet conditions for infection and symptom development and hence is associated with rainy weather. Splashing water moves microsclerotia and conidia from soil onto leaves, resulting in infection.
To manage anthracnose, first avoid planting lettuce in fields having a history of the disease. Use irrigation systems (furrow or drip irrigation) that reduce or eliminate splashing water and leaf wetting. Apply protectant fungicides, such as strobilurins, which are effective for controlling this disease.

Photo 1. Bacterial leaf spot results in black, angular shaped lesions on lettuce leaves.
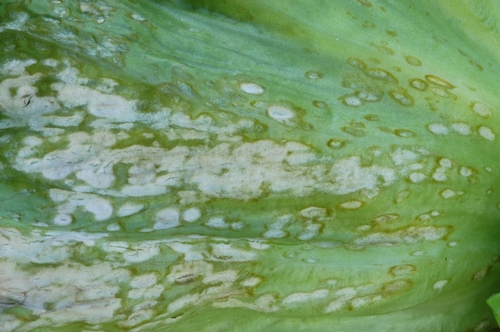
Photo 2. On lettuce, anthracnose results in tan, angular shaped lesions that are covered with the white to pink growth of the fungus.
- Author: Jim Correll, University of Arkansas Steven Koike, University of California Cooperative Extension
Another new race, the 13th, of the downy mildew pathogen (Peronospora farinosa f. sp. spinaciae) of spinach has been found and documented. First identified in January 2010 from spinach in Holtville, California, this race breaks the resistance of several important cultivars. The isolate was initially designated as UA0510C and was characterized with a standard set of differential varieties. Isolates apparently identical to UA0510C have been found in an increasing number of locations throughout California in 2010 and 2011. After careful evaluation of the significance of this development to the spinach industry, the International Working Group on Peronospora (IWGP) has designated this isolate as race Pfs 13. The IWGP is located in The Netherlands and is administered by Plantum NL.
Race Pfs 13 poses a threat to the spinach industry because it is particularly well-adapted to modern hybrids with resistance to races 1-12. The appearance of a new race is not unexpected because hybrids with resistance to races 1-12 have been widely planted over the past few years. Similar developments have taken place when races Pfs 5 (1996), Pfs 6 (1998), Pfs 7 (1999), Pfs 8 and 10 (2004), Pfs 11 (2009), and Pfs 12 (2009) were identified and named. The occurrence of Pfs 13 will clearly encourage the industry to develop and use new spinach cultivars having resistance to races 1-13. A history of the detection of the various spinach downy mildew races is presented in Table 1.
A collaboration of researchers with the IWGP, University of Arkansas (Correll), and University of California (Koike) is monitoring the development of new races of spinach downy mildew on a global scale by collecting and testing suspected new isolates. In this way it is hoped that research findings and conclusions will be agreed upon and better communicated between the seed industry, spinach growers, and other interested parties. For California and Arizona, the Correll-Koike team will continue to receive and test spinach downy mildew samples for growers, pest control advisors, and seed companies. Industry is encouraged to continue to submit downy mildew outbreak samples to Correll-Koike, as such samples facilitate the discovery of additional new races. The Correll-Koike research is made possible by support from the California Leafy Greens Research Board and by active participation by the agricultural industries in California and Arizona.
For more information on this subject you can contact Steven Koike (stkoike@ucdavis.edu), Jim Correll (jcorrell@uark.edu), Diederik Smilde (d.smilde@naktuinbouw.nl), or IWGP chairperson Jan de Visser (JandeVisser@popvriendseeds.nl).
Table 1. Races of spinach downy mildew and year of detection
| Year | Race |
| 1824 | 1 |
| 1958 | 2 |
| 1976 | 3 |
| 1990 | 4 |
| 1996 | 5 |
| 1998 | 6 |
| 1999 | 7 |
| 2004 | 8 |
| ... | (9)* |
| 2004 | 10 |
| 2008 | 11 |
| 2009 | 12 |
| 2010 | 13 |
*One time detection only

Downy mildew of spinach is the most important disease on this crop and results in quality and yield losses.
- Author: Jim Correll
- Author: Steven T. Koike
Yet another new race of downy mildew (Peronospora farinosa f. sp. spinaciae) on spinach has been identified in California’s Salinas Valley. The type, or original, strain was initially designated as UA2209 and was first detected in May 2009. Subsequently, it was found in an increasing number of locations throughout California in 2009 and 2010. This race breaks the resistance of several important cultivars. The race has been characterized on a set of differential cultivars and was designated as race Pfs 12 by the International Working Group on Peronospora (IWGP). The working group is located in the Netherlands and is administered by Plantum NL.
Race Pfs 12 poses a threat to the spinach industry because it is particularly well-adapted to most modern hybrids with resistance to race 1-11, which have been widely planted in the past few years. Race 12 is distinct from race 11 because of its virulence on the differentials Campania and Avenger. The appearance of a new race is not completely unexpected because hybrids with resistance to races 1-11 have been planted on a large scale. Similar developments have taken place when races Pfs 5 (1996), Pfs 6 (1998), Pfs 7 (1999), Pfs 8 and 10 (2004), and Pfs 11 (2009) were identified and named. The occurrence of Pfs 12 will create strong interest for Pfs 1-12 resistant spinach cultivars from both growers and breeders.
The IWGP is a working group of Plantum NL consisting of spinach seed companies (Pop Vriend, Monsanto, Rijk Zwaan, Nunhems, Takii, Sakata, Bejo, Enza, Syngenta, Advanseed), Naktuinbouw, and the University of Arkansas. The efforts of the group are supported by research activities at the University of Arkansas and the University of California Cooperative Extension—Monterey County. The aim of the IWGP is to monitor and designate new races of downy mildew in spinach, and to promote a consistent and clear communication between the seed industry, researchers, and growers about all resistance-breaking races that are persistent enough to survive over several years, occur in a wide area, and cause a significant economic impact.
IWGP is monitoring new races continuously by testing field isolates on a fixed, common host differential set of cultivars that contains the full range of available resistances. Researchers all over the world are invited to join the IWGP initiative and use the common host differential set to identify new isolates. For California, the Correll-Koike team will continue to receive and test spinach downy mildew samples for growers, pest control advisors, and seed companies.
For more information on this subject you can contact Steven Koike (stkoike@ucdavis.edu), Jim Correll (jcorrell@uark.edu), Diederik Smilde (d.smilde@naktuinbouw.nl), or IWGP chairperson Jan de Visser (JandeVisser@popvriend.nl).

Downy mildew is the most damaging disease of spinach in California and causes yellow and tan leaf lesions.

To identify downy mildew races, a series of spinach cultivars is grown and inoculated; races are identified based on which cultivars become diseased.






