Pre-bloom foliar boron application on olive
may improve yield
Ellie Andrews, PhD, UCCE Farm Advisor, Sonoma County, and Elizabeth Fichtner, PhD, UCCE Farm Advisor, Tulare County
Olive orchards entering an OFF year in 2024 may benefit from pre-bloom foliar boron (B) applications to support reproduction and yield. Because the 2023 California olive crop varied widely both within and between olive-growing regions, the value of boron applications should be considered at the individual orchard level. For example, in the southern San Joaquin Valley, the 2023 ‘Manzanillo' table olive crop was OFF due to the high temperatures at bloom whereas many oil cultivars in the region were unaffected by the heat and had heavy production. Those orchards that had a heavy ON crop in 2023 may benefit from pre-bloom boron application in the 2024 season.
Boron is an essential micronutrient for plant growth and reproduction. Boron deficiency affects plant reproduction by reducing pollen viability and germination and limiting pollen tube growth. Deficiency also limits the proportion of flowers that set fruit and reduces the retention of developing fruit. The influence of boron deficiency on multiple stages of reproduction may negatively impact yield. Boron also plays a role in vegetative growth and metabolism, ensuring cell wall and membrane integrity and facilitating sugar transport and cell division. Because boron plays a crucial role in reproduction, boron is translocated from vegetative tissues to reproductive tissues resulting in higher concentrations of the nutrient in reproductive organs than leaves. Due to this high demand, reproductive boron deficiency can occur even when vegetative boron and available soil boron are sufficient.
Studies conducted across numerous global olive-growing regions demonstrate the beneficial effects of foliar boron application on yield, particularly in advance of an OFF crop. The influence of boron application on productivity in olive orchards may relate to increases in photosynthesis, an increase in the number of perfect flowers (those with both male and female reproductive parts) (Figure 1), and an increase in pollen viability, or pollen tube growth. Olives are considered andromonoecious, a reproductive strategy in which plants bear both hermaphroditic (perfect) flowers and male flowers. Stress prior to bloom may cause pistil abscission in a fraction of buds resulting in a higher percentage of male flowers. Several research studies have demonstrated that pre-bloom foliar boron application can increase the percent of perfect flowers on trees, thus increasing the number of flowers capable of producing fruit. In olive, boron is readily mobilized from both young and old vegetative growth to support flower and fruit production; therefore, a portion of boron applied throughout the year may be utilized to support reproductive processes. During the pre-bloom season, however, cool temperatures and the corresponding reduced physiological activity may limit the uptake and translocation of boron in olive. Additionally, flowers are not as strong a boron sink as fruit; therefore, the pre-bloom foliar application may render the micronutrient available at a short-lived, yet critical, time in crop development.
Both oil olives and ‘Manzanillo' table olives have been shown to benefit from foliar boron applications. For example, ‘Arbequina' receiving pre-bloom foliar application of boron exhibited increased bloom and a 27% increase in yield in an OFF year. In the ‘Arbequina' study, no value of boron was observed in an ON year, and boron was found to have no effect on vegetative growth. In another study, boron applications to ‘Frantoio' resulted in increased concentration of chlorophyll and soluble sugars, as well as changes in the profile of endogenous plant growth regulators within the leaves. In California, pre-bloom boron applications on ‘Manzanillo' resulted in increased percentage of perfect flowers and improved fruit set and yield, particularly during an OFF year.
The recommended foliar boron concentration for olives ranges from 19-150 ppm. Values below 14 ppm boron may result in boron deficiency, whereas values above 185 ppm may result in boron toxicity. A foliar nutrient analysis only provides a snapshot of the status of the plant at the time of leaf collection; however, low boron status of leaves has been found to correlate well with symptoms of deficiency. Symptoms of boron deficiency in olive include dead leaf tips with a characteristic yellow band and green leaf base, as well as twig and limb dieback (Figure 1). Boron deficiency may first become apparent in the meristems, the growing tips of shoots. Boron deficiency may also result in misshapen and defective fruit (Figure 1), low fruit set, and premature fruit drop. The value of boron application for improved fruit set is not limited to orchards with visual symptoms of boron deficiency or foliar boron levels below the recommended range. In fact, the numerous research studies that demonstrate the value of pre-bloom foliar boron applications for enhanced fruit set and yield were conducted in orchards with no boron deficiency. Based on these findings, foliar analysis alone may not be a useful predictor of benefits from pre-bloom foliar boron application.
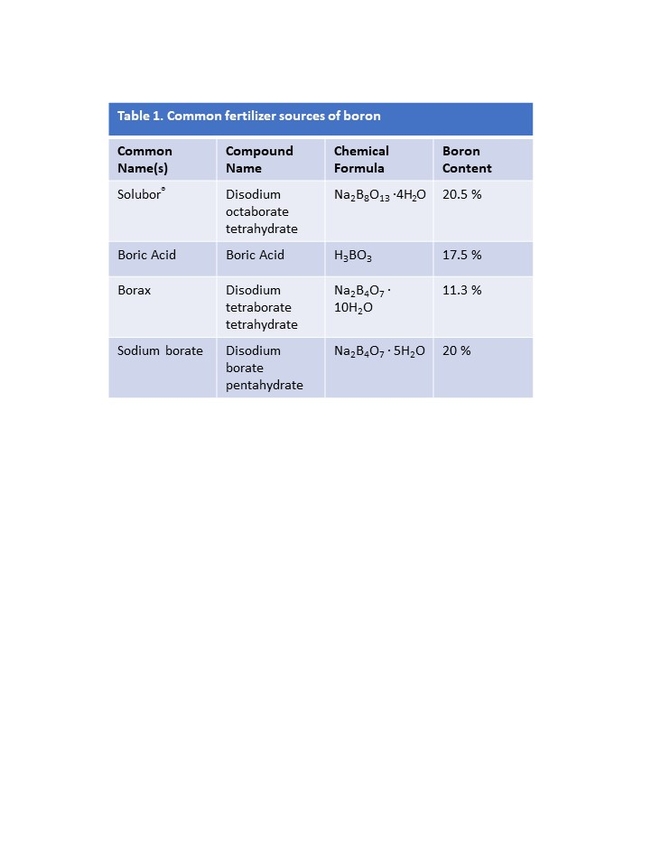
Boron is typically introduced to orchards either as a solid mineral broadcast on the soil surface, or in solution as a foliar spray. The pre-bloom foliar application is designed to specifically enhance fruit set and yield and should be applied three weeks prior to bloom. Boron is generally sold as borax, sodium borate, sodium tetraborate, boric acid, or Solubor® (Table 1). The boron content varies between formulations; therefore, all calculations should be based on the equivalents of active ingredient (ie. pounds of boron). For example, for soil-applied boron in olive, 5-10 lbs/acre of boron is broadcast, which equates to approximately 45-49 lbs/acre of borax (11% boron) or 24-48 lbs/acre Solubor® (20.5% boron). In California, foliar application of boron three weeks prior to ‘Manzanillo' bloom, particularly in OFF years, at rates of 1 or 2 lb./acre Solubor® in a 100 gallon/acre (246 or 491 mg/L boron at 935 L/hectare) was demonstrated to improve yield by approximately 30%. The baseline boron level in this California study site was 16 ppm boron, a level just below the established critical level, but high enough to avoid deficiency symptoms.
The value of boron applications on orchard health and economic return varies based on the status of the alternate bearing cycle in the year of application, the baseline boron status of the tree and soil, and other climate factors that may influence yield. Plants have a narrow range between boron deficiency and toxicity. Be sure to read the product label carefully to avoid over-application and conduct annual leaf tissue analyses to gather baseline information on the boron status of orchards. More information on fertilizer rates for olives and other California crops may be found on the CDFA FREP California Crop Fertilization Guidelines website (https://www.cdfa.ca.gov/is/ffldrs/frep/FertilizationGuidelines/).
- Author: Ben Faber
This little mnemonic, or memory aid, in the title is helpful in remembering the critical levels of toxic constituents in irrigation water. The “one” stands for 1 part per million (ppm) of boron (B), the e” hundred” flags 100 ppm of sodium (Na) and (Cl) and the “thousand” represents the level of total soluble solids (TDS or slats) in water. Levels exceeding the critical values for any of these constituents can present problems for tree growers. The problems typically show themselves as tip-burn and defoliation. The B, Na and Cl are toxic elements at relatively low concentrations, but symptoms appear similar to the damage caused by high salinity.
Water that exceeds the critical levels mentioned in the mnemonic has a greater tendency to cause damage if sufficient leaching is not applied. It doesn't mean the water is impossible to use, only that greater attention needs to be made to ensure that these salts are adequately leached. High levels of these salts accumulate in the soil with each irrigation, and the salts are absorbed by the tree and end up in the leaves where they do their damage.
This promises to be another low rainfall year and the customary leaching we rely upon in winter rainfall is not going to be as effective as in customary years. Irrigation is a necessary evil. Every time we apply irrigation water we apply salts, and unless some technique is used to minimize salt accumulation, damage will result. This damage can be more than just leaf drop, but also the stress that induces conditions for root rot.
Irrigation water has been applied the last four years and many trees looked stressed. Even well irrigated orchards have leaf burn due to the gradual accumulation of salts from irrigation. It is probably necessary to irrigate in many winters. With the lack of rain problem, it may be necessary to irrigate even if there is rain. The wetted pattern that is created by a drip or microsprinkler emitter also creates a ring of salt in the outer band of the wetted patter. If there is less than an inch of rainfall to push this salt down, this salt tends to diffuse towards the tree where it can accumulate back in the root system. Orchards with even good water quality would find it advisable to run the irrigation system with the first rains. Growers with water quality exceeding one, hundred, or thousand should be especially alert to the need to manage water in low rainfall years.

- Author: Ben Faber
Assessing water quality for Southern California agriculture typically revolves around the total salinity of the water, its total dissolved solids (TDS), and the toxic ions boron, sodium and chloride. Salts are necessary to plants, because it is in the form of diluted salts that all nutrients are taken up by plants- the macro and micronutrients plants extract from the soil. High salinity leads to water imbalance problems much as if the plant were not getting adequate water. A toxicity problem is different from a salinity problem, in that toxicity is a result of damage within the plant rather than a water shortage. Toxicity results when the plant takes up the toxic ions and accumulates the ions in the leaf. The leaf damage that occurs from both toxicity and salinity are similar in that it causes tissue death known commonly as "tip burn." The damage that occurs depends on the concentration of the ions in the soil water around the roots, the crop sensitivity and crop water use, and the length of time the crop experiences the ions. In many cases, yield reduction occurs. Because crops can not excrete salts the way humans do, salts gradually accumulate in a plant. As a result plants need a higher water quality than humans do.
Much study in many countries has gone into evaluating water for crop use. Some of these studies have been on the effects of salts on soil characteristics. Generally, as sodium concentration increases, a soil will lose its aggregation, eventually leading to poor water infiltration. Many more salinity and toxicity studies have been done on plants themselves. Not all crops are equally tolerant of salinity and toxicities, and in general most plants respond to salinity and toxicities in a similar fashion. If a plant is intolerant of salinity, it will be intolerant of chloride, sodium and boron. Most annual crops are less sensitive to salts than tree crops and woody perennials, although symptoms can appear on any crop if concentrations are high enough. The reason for greater sensitivity for perennial crops is that the tree is sitting in the ground absorbing salts for a longer period than the lettuce plant that is harvested 3 months after planting. Furthermore, deciduous trees like walnut shed their leaves each winter, so they can handle salinity better than evergreens like citrus and avocado.
To manage salinity and toxicities, water management is the key. Depending on water quality, an excess of water will be applied to the soil to leach the previously applied salts away from the root zone. The poorer the water quality, the more excess water is applied.
Selecting a less sensitive crop is also an alternative when dealing with poor water quality. Some barley varieties can handle salinity similar to ocean water. Barley nets a grower $400 an acre, avocados $9,000 and $25,000 if the market is right for strawberries. Avocados are salt sensitive, so are strawberries and lemons and cherimoyas and star fruit and blueberries and raspberries and mandarins and nursery crops. We grow these because with our climate, very few other places can grow them and they return enough money for a grower to stay in business in an area where land, water and labor are expensive. We really don't have much in "alternative crops" to grow here.

- Author: Ben Faber
Along with drought there are also concerns about water quality which has all kinds of weird units that area actually convertible. Here's a little guide for the principle water quality components and their conversions.
Water Terminology
Common ions in water: calcium (Ca2+), magnesium (Mg2+), sodium (Na1+)
sulfate (SO42-), chloride (Cl-), carbonate (CO32-), bicarbonate (HCO3-), boron (H3BO3)
Measured as parts per million (ppm) or milligrams per liter (mg/l), which are interchangeable , or milliequivalents per liter (meq/l). A milliequivalent is the ppm of that ion divided by its atomic weight per charge.
Example: Ca2+ with atomic weight of 40 and a solution concentration of possibly 200 ppm. Ca2+ has two charges per atom, so it has a weight of 20 per charge. 200 ppm divided by 20 = 10 meq of calcium for a liter of water.
Total Dissolved Solids (TDS): measure of total salts in solution in ppm or mg/L
Electrical Conductivity (EC): similar to TDS but analyzed differently.
Units: deciSiemens/meter(dS/m)=millimhos/centimeter (mmhos/cm)=
1000 micromhos/cm (umhos/cm).
ConversionTDSEC: 640 ppm=1 dS/m= 1 mmhos/cm=1000 umhos/cm
Hardness: measure of calcium and magnesium in water expressed as ppm CaCO3
pH: measure of how acid or base the solution
Alkalinity: measure of the amount of carbonate and bicarbonate controlling the pH, expressed as ppm CaCO3.
Sodium Adsorption Ratio (SAR): describes the relative sodium hazard of water
SAR= (Na)/((Ca+Mg)/2)1/2, all units in meq/l
1.5 feet of water with EC of 1.6 adds 10,000 # of salt per acre
and that same water with 20 mg/l of nutrient will supply 80# of that nutrient/acre
Sea water has ~ 50 dS/m, 20,000 ppm Cl, 10,000 ppm
Irrigation water WATCH OUT- 1,000 ppm TDS, 100 ppm Na/Cl, 1 ppm B

- Author: Craig Kallsen
Many citrus trees in the southern end of the San Joaquin Valley are grown on moderately calcareous soils and frequently have high levels of boron in the leaf tissue. Citrus is sensitive to boron. Boron, when excessive, may cause defoliation and significant yield loss. At high, but nontoxic concentrations, leaf symptoms are similar to those caused by excessive salt, deficient potassium, heat stress, or biuret toxicity from urea foliar sprays. Therefore a leaf tissue analysis is important for delineating causes.
Excessive levels of boron produce a yellowing of the tip of leaves and yellow spotting of the leaf surface. Death of the leaf tissue may occur along the margins. Higher levels of boron may cause brownish, resinous gum spots on undersides of leaves but this symptom is not always present. Leaf symptoms are most severe on the “hot” south side of the tree. Boron accumulates in the leaves as they age so symptoms usually appear on older leaves first. Older leaves with high concentrations of boron are relatively short lived compared to trees that have boron at optimum concentrations. Often excessive boron and sodium appear together in leaf tissue analyses. Boron is associated with a decreased distance between leaf nodes. Trees with high leaf tissue boron concentrations appear to be less vigorous with shorter branches, probably as a result of the association of boron with decreased distance between leaf nodes.
Discussion of levels of boron which would be considered excessive in September-sampled spring-flush leaf tissue may be misleading because the particular leaves that are selected for the sample can greatly influence results. If only leaves with the most severe symptoms are sampled, such as leaves that are mostly yellow with dead margins, concentrations of boron can reach into the thousands of parts per million (ppm). A truer picture of the boron status of the grove can be gained by pulling leaves with ‘average’ symptoms. Using this sampling technique, the highest level of boron in orange leaves seen in this office over the past eight years has been 600 ppm from an isolated and particular calcareous part of an orchard located near the town of Edison in Kern County.
Standards from citrus in Florida for the concentration of boron in leaf tissue (4-6 month old leaves on nonfruiting terminals) correlate well with observations made in the San Joaquin Valley as follows:
Deficient <20
Low 21-35
Optimum 36 - 100
High 100 - 200
Excess > 250
Leaf boron concentrations greater than 250 ppm are excessive, but in older orange, lemon and grapefruit trees visible leaf symptoms are not usually manifested until leaf-tissue boron concentrations exceed 300 ppm. A range of 300 to 400 ppm show little outward sign of boron toxicity except for some slight tip yellowing and some reduction in vigor. Excessive defoliation does not usually begin in most citrus until concentrations of approximately 450 ppm are reached. Trees at 450 ppm and greater will, generally, exhibit a thin-canopied, unthrifty, somewhat stunted appearance. The yield of the tree does not appear to be affected nearly as rapidly as the appearance of the canopy. At least one large lemon grove in Kern County, that characteristically produces excellent yields of early-maturing, good quality fruit, has elevated leaf-boron levels. Moderate levels of leaf boron, in the 300 to 400 ppm range in this orchard appear to reduce tree growth, reducing the need to prune, while yield remains relatively unaffected.
Leaf boron concentrations greater than 300 ppm probably warrant further investigation as to the source of the boron. Orange leaf tissue samples taken from trees planted in the 1960’s or early 1970’s in Kern County routinely show levels of 300 to 400 ppm. Young trees appear to increase in boron concentration rapidly but at about 300 to 400 ppm the concentration tends to plateau. Why boron levels tend to plateau is not known. Chandler pummelos appear to be the most sensitive to excess boron, followed by lemons, grapefruits and oranges. Leaf boron concentrations of 400 ppm in Chandler pummelos appear to have caused severe stunting of the trees in several orchards in Kern County, while similar levels in Melogold (a pummelo x grapefruit hybrid) resulted in only some tip burn.
There are actions the grower can take to reduce the amount of boron in the tree. First the source of the boron should be determined if possible. If boron levels are increasing in the leaf tissue, analyze both surface water and well water. Avoid using water with greater than 0.5 ppm of boron for irrigation of citrus. Levels of boron that are beneficial to cotton or pistachio can cause severe problems with citrus. Surface water comes from diverse sources in Kern County. Surface delivered water may have started out as well water, or in some instances as diluted oil-field waste water which may contain relatively high concentrations of boron. Water districts will know if oil-field waste water is being diluted in irrigation water. Use of oil-field waste water can be seasonal and irrigation derived in part from oilfields may fluctuate in boron concentration. If boron is in the water even at slightly elevated levels, avoid spraying it directly on the trees when treating for insect pests or when applying foliar fertilizers. Fertilizers are foliarly applied because of the quick uptake of dissolved minerals through the leaves. If boron is in the spray solution, it will be absorbed quickly by the tree along with the potassium, zinc, manganese, nitrogen and other foliar nutrients. Organic matter, manure, composted materials, and mulches on the ground have been shown to reduce boron uptake by the plant from irrigation water with high concentrations of this element.
In the southern San Joaquin Valley, soils should be tested before citrus is planted. Areas of soil with high boron are found in the most unexpected places. Boron may have accumulated on some properties when high-boron well water was used before the advent of easier access to water from Sierra snow melt.
If leaf-tissue boron is high and the water or soil is not, check the foliar fertilizer blends being used. Often, boron is included in many micronutrient mixes because boron can be deficient in acid soils. Determine how much boron soil amendments may contain. Pit gypsum can have varying quantities of boron in it. A ton of this gypsum may contain as much as 20 pounds of boron.
Discovering the cause of high boron in citrus leaves may require an extra soil test in addition to the typical saturated pest extract. Soil tests for ‘available’ boron using a saturated pest extract can be deceiving. In many instances where the concentration of boron in a ‘typical’ leaf averaged greater than 300 ppm, plant-available boron in the soil and water frequently averaged less than 0.25 ppm. However, total soil boron in these same orchards was at very high levels. Total soil boron estimates both available and unavailable boron. To help determine where the boron in the trees originates, both readily available and total soil boron should be sampled. This disparity between plant-available and total boron suggests that boron moves between the relatively small plant-available pool in the soil and the much larger ‘unavailable’ pool tied up in these calcareous soils. Soil acidifying agents and acid-forming fertilizers probably increase the availability of boron to citrus trees by making boron that is relatively unavailable to the trees at high pH, more available at lower pH. At any given time, plant-available boron may be relatively low but its constant replacement from the unavailable pool keeps the boron concentration in trees relatively high. In orchards where total soil boron is elevated; soil pH should probably be kept as high as tree health permits. Where the total amount of soil boron is moderate and soils are relatively well-drained and topography is flat, acidifying and leaching is probably the preferred option for reducing boron levels. Acidifying the soil and not supplying sufficient water to leach the boron from the root zone can compound the problem by making more boron readily available to the tree.
If boron is not found in the upper soil profile, but is found or suspected to exist deeper, irrigations could be scheduled that are more frequent but of shorter duration so that most of the citrus roots remain in the upper, lower-boron portion of the soil profile.
Actively growing, vigorous trees may dilute the concentration of boron in the leaf tissue through the production of a thick canopy. Old leaves tend to accumulate boron and drop. Adequate nitrogen ensures that enough nitrogen is present for production of new leaves. Increasing the nitrogen fertilization rate can encourage vegetative production and enhance this effect, but too much nitrogen may be associated with adverse fruit quality characteristics like regreening of Valencias, later maturity of early navels or higher yields of smaller fruit. Keeping other nutrients in the leaf in balance is important if boron is present at excessive concentrations. Maintaining high concentrations of phosphorous and calcium in the leaves through an appropriate fertilization program should be beneficial as these nutrients have been shown to reduce absorption of boron.