- Author: Ben Faber
With little rain this winter and the erratic weather patterns of wind and heat, avocado is going to be especially prone to salt damage. And the flowering period is one of the most sensitive. Flowers are competing with leaves that have been hanging on for a year and have been salt stressed by a year's worth of irrigation salts. A good understanding of how salt moves and is leached it important to get through til next winter when there will hopefully be sufficient rain again to naturally leach the soil.
Water moves in a wetting front. When irrigation water hits the soil, it moves down with the pull of gravity and to the side according to the pull of soil particles (more lateral with more clay). Soil is a jumble of different sized soil particles, from clay to silt to sand sizes and then often intermixed with stones of different sizes from gravels to boulder. The different textures determine how water moves. It moves fastest through coarse textures and slowest through finer ones – the clays, the ones with the smallest pores. But soils are a jumble of particle sizes and pores.
Water first moves down the larger pores and then it slowly moves through the smaller ones. As water moves through the soil, it carries salts that have accumulated in the soil. At the wetting front is where the salt accumulates. As the water moves through the larger pores, salts migrate/diffuse from the small pores to the larger ones. This diffusion takes a bit of time, so typically the small pores have a larger salt concentration than the larger ones.
So an initial application of water will carry the salts from these large pores and if the irrigator were to stop in mid-application, it allows time for the salts to move out of the small pores into the larger ones. Then when the irrigation recommences, it will carry more of the salts out of the wetted area – the root zone. This technique is called “bumping” where an irrigation is stopped and then restarted in order to improve not only leaching, but also reduce runoff.
This principle also is at play when there are two or more sources of water quality. Soil salinity can be no lower than the irrigation water that is applied. Then as the soil, water is removed through plant absorption or evaporation, the salinity increases. The soil salinity can easily be two to three times higher than the irrigation water.
If there are two sources of water, the initial application can be with the poorer quality water, and once that has reduced the soil salinity, then the better water quality can be applied which will then bring the soil salinity closer to that of the better quality water. By doing this two part leaching, the amount applied of the better quality water can be significantly reduced. This is a type of “bumping” to improve leaching.
Watch this U-Tube video on how water moves through soil, thanks to the work at Walla Walla Community College.
https://www.youtube.com/watch?feature=player_detailpage&v=J729VzBeI_g
Thank you Walla Walla Community College for the video

- Author: Ben Faber
Irrigation volume and frequency: soil, salinity and nutrient considerations
- Author: Mark Battany
Published on: July 18, 2019
Irrigation frequency and volume
One fundamental decision that a grower needs to make is how frequently to irrigate a vineyard; either applying small amounts of water frequently, or larger amounts of water less frequently. This choice determines how large the soil "flower pot" is that supports the vines, while also having implications for nutrient availability, salinity conditions and potential limitations on water infiltration. Changing from frequent small irrigations to infrequent large irrigations, either as an ongoing practice or as a one-time event, may lead to unanticipated outcomes and thus should ideally be done after ensuring that the conditions are adequate for this practice. For this reason it can be beneficial to evaluate the soil and water quality conditions at a site before making large changes in irrigation practices. Factors to consider include the potential depth of the rootzone, the presence of any layers in the soil which may cause infiltration problems, the salinity of the irrigation water, and the potential nutrient conditions affected by changing the wetted soil volume.
Root zone depth
If applying a large volume of irrigation water, the soil needs to have the capacity to store this water while providing adequate porosity conditions allowing gas exchange for proper root function. The soil depth to bedrock needs to be considered; if this depth is shallow in areas of the vineyard, this can lead to poor performance with large irrigations if they result in ponded water above the bedrock or large variations in total available soil water due to varying soil depths.
Figure 1. An example of shallow bedrock underlying an otherwise productive soil.
Most of our vineyards are located on deeper alluvial soils where shallow bedrock is not a concern. For a given rooting depth, finer-textured soils with their relatively high water holding capacity can store more water and thus be irrigated with larger volumes less frequently, while coarse-textured soils with their lower water holding capacity generally need to be irrigated with smaller amounts more frequently. Grapevine roots can grow very deeply in the soil, more so if that is where available water is found; however a vine which has developed mostly shallow roots from a history of shallow irrigation may not be able to take advantage of recently applied deeper soil moisture until it has developed the roots to do so.
Less permeable layers
Common throughout the Central Coast are different types of low-permeability layers in soils which can impede the movement of water, resulting in ponding or saturated conditions above the restrictive layer with negative impacts on any roots in that zone. These less permeable layers may not have been been given much attention until well after a vineyard has been planted, for example when mature vines are observed to suffer stress in heavy rainfall years or with a change to longer duration irrigation in the summer. A thorough evaluation of a site prior to planting should include an assessment of the deeper soil conditions to identify any potential restrictions on the movement of water or penetration of roots. Sites which have such conditions that cannot be corrected are not good candidates for applying large volumes of irrigation water in the summer, if doing so results in water saturation of the active root zone.
Impermeable layers can exist due to a variety of physical and/or chemical conditions in the soil. A hardpan can be formed naturally in the soil due to the gradual compaction and cementing together of soil particles. Agricultural practices of using moldboard plows and heavy equipment can also create hardpan conditions.
Figure 3. This hardpan layer became evident when irrigation volumes increased, and vines collapsed.
A clay lens is a horizontal layer of clay in between soil layers; this clay can be an effective barrier to both water movement and root penetration. These clay lenses can occur below the depths which can be effectively corrected with tillage, leading to perched water tables which can be particularly troublesome by preventing deeper drainage.
Another type of textural barrier occurs in a stratified soil, when a fine-textured soil horizon overlies a coarse-textured horizon. Water does not flow downward out of the fine-textured horizon and into the coarse-textured horizon until the former is fully saturated with water. This may seem counter intuitive, because we generally associate coarse soils with good drainage; in reality this stratified condition results in water-logging of the fine soil layer when large amounts of irrigation are applied. With small volumes of irrigation this fine soil layer may be wetted enough to support the bulk of the vine roots; a subsequent change to a large volume of irrigation can saturate this same soil and negatively affect root function.
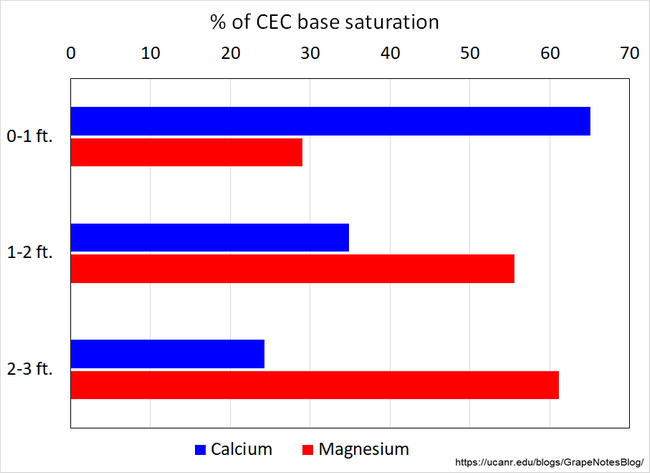
A less-permeable layer can also be due to variations in soil structure where the cation exchange capacity of the deeper soil is dominated by magnesium, while the surface horizon has more calcium, typically from the historic additions of lime or gypsum amendments. The greater calcium content of the surface horizon creates a more porous and stable soil structure that allows for good water penetration, whereas the higher magnesium content of the deeper soil results in a very dense, massive soil structure with much less ability to infiltrate water.
Chemical weathering of the soil can also form low-permeability layers over thousands of years. An example are the "calcic" (also known as "caliche") soil horizons which are formed by rainfall dissolving the naturally occurring lime in the upper soil horizons, which then moves downwards where it precipitates out of solution (becomes solid) again at a deeper depth. Because the precipitation of the lime occurs within the existing soil pores, this process gradually clogs these soil pores and creates a barrier to water movement and root penetration.
Figure 7. A calcic horizon about two feet below the surface; east of Paso Robles.
The above types of restrictive layers may exist in current vineyards, and they can be very difficult or impossible to alter with the vines in place. These restrictive layers are more effectively addressed during the vineyard development phase, when deeper tillage is possible. Some conditions such as the presence of highly stratified layers can be very difficult to correct and require the use of very large equipment, which will forever alter the natural state of the soil.
Such extensive deep tillage may not be desirable or feasible for a variety of practical and philosophical reasons; in that case the irrigation management needs to be adapted to the presence of these soil barriers to water movement. Lighter, more frequent irrigation can avoid problems due to deeper restrictive layers. Alternatively, applying irrigation more broadly by increasing the number of emitters per vine (two 1/2 gph emitters instead of one 1 gph emitter for example) can increase the wetted soil volume while avoiding the potential deeper problem layers if applied volumes remain small. A heavy summer irrigation which results in the extended saturation of the soil horizon containing the most active roots can result in vine collapse and death, but the same soil conditions during the winter will not impact the vines in the same manner; thus applying large irrigations to soils during the winter to increase their water storage can be more successful than during the summer when roots are active and the vine root water uptake is at its maximum. This type of winter irrigation is more useful in dry regions where rainfall is limited, and may not be suitable for areas that receive heavier precipitation.
Soil salinity considerations
Another factor to consider when determining the irrigation frequency is the potential for soil salinity to impact vine growth. Groundwater quality throughout the Central Coast is highly variable; sites with relatively poor-quality irrigation water, particularly those having rootstocks susceptible to salinity, need to take this into consideration when choosing their irrigation frequency. To understand why, consider this example: after applying irrigation, assume that the electrical conductivity of the soil water is the same as the irrigation water. As the soil water is consumed by root uptake and evaporation, the volume of soil water is reduced but most of the salts remain behind in the soil water. As a result, the concentration of salts in the remaining soil water gradually increases as the volume of soil water decreases. This adds an additional stress on the vines, an osmotic stress, which reduces the vine's ability to take up the remaining soil moisture. We can minimize this osmotic stress by increasing the frequency of irrigation, which ensures that at least a small volume of the soil is maintained at a higher water content and therefore a lower salinity level. With higher frequency irrigation, the vines won't experience the same degree of increasing salinity stress towards the end of the irrigation cycle as they would between large, infrequent irrigations. This characteristic of high-frequency drip irrigation to maintain a lower osmotic stress is what has permitted successful crop production where conventional irrigation would not be feasible due to the poor quality irrigation water.
Figure 9. Severe water stress, and potentially salinity stress as well.
Nutrient considerations
The choice of whether we are growing the vines in a "big pot" or a "small pot" has important implications for nutrient management as well. When a larger volume of soil is wetted with irrigation, this can increase the total amount of soil nutrients which are available to the vines; this can be good or bad, depending upon the situation.
As the soil dries out over the summer and early fall, the most active roots will be in the volume of soil wetted by irrigation. If this soil volume does not contain sufficient nutrients, then deficiencies can occur. A common example is potassium; it can exist in adequate quantities in the drier soil outside the wetted volume but is not readily available to the roots under these dry soil conditions. This is an example of an induced deficiency, where the nutrient is present, but conditions do not permit its uptake by the vine. This condition is typically addressed by fertilizing with the nutrient in the wetted soil volume, generally by fertigation. Increasing the wetted soil volume can allow the vines to access nutrients which previously were not as accessible such as the potassium example above. It may also increase the availability of other nutrients, for example nitrogen which had leached below an earlier shallow root zone. An increased level of available nitrogen may lead to excessive vegetative growth, thus these deeper nutrient levels may need to be evaluated before increasing the soil wetted volume.
Summary
Prior to making major changes in the irrigation frequency and amounts at a site, the soil and rooting conditions should be evaluated to predict whether or not such changes might have any negative effects on the growth of the vines. The factors involved are relatively straightforward but can be difficult to evaluate due to the need to dig deeply in the soil, and often at multiple locations if there is much variability at the site. Making this effort can help predict what types of changes may occur due to alternations in the irrigation patterns, and help identify situations beforehand that could result in undesired outcomes.
Videos of water movement in soils
Water Movement in Soils, a classic 1959 video produced by Washington State University has some excellent demonstrations of how water moves in soils; some key examples:
A coarse soil layer underlying a fine soil layer:
https://youtu.be/DmTNFIEc2VA?t=227
A clay layer (which will behave similarly to a hardpan layer or a strong calcic horizon):
https://youtu.be/DmTNFIEc2VA?t=395
A more recent video from the University of Arizona has similar demonstrations of water movement in stratified soils:
https://youtu.be/Ph-7tQuIbz4?t=899
A historical perspective on hardpan soils
For a history of local farmers dealing with hardpan soils on the Central Coast a century ago, see the earlier blog article: The rise (and demise) of the UC Experiment Station at Paso Robles
- Author: Ben Faber
Irrigated agriculture must always contend with salts. Five years of drought and its effects can magically disappear, but it will be back again. Low rainfall is the norm for California. We rely on winter rainfall to leach the salts from root zones that have accumulated salts from previous irrigations. Salinity affects plant growth and understanding what it is and how it is measured and evaluated need to be understood. Just having wet soil that is full of salts is not going to help a plant, it's going to add stress and eventually physiological and disease problems - https://ucanr.edu/blogs/Topics/index.cfm?start=28&tagname=disease
All waters, even rain water, have some salts dissolved in them, so all waters could be called saline. The term saline is restricted to waters with concentrations that could cause harm to plants or people. Seawater is highly saline, many wells are moderately saline. But unlike humans that excrete salts, plants are often affected by salt levels that have very little health impact on humans. Well waters used for irrigation can often exceed standards for plants that are fit for human consumption. However, with proper management many waters can be used on plants, depending on the plant species. Domestic water supplies from cities typically have better quality than some well waters because they are monitored and often blended to meet human consumption. Most domestic water supplies have low concentrations of salts and are not considered to be saline. However, using even domestic water in growing subtropicals does not mean that we should not be concerned about salinity.
Before going any further it is worth remembering that salt is not just the sodium chloride that's on the table. Salts are combinations of electrically charged ions. These ions separate from one another when a salt dissolves in water. Water with dissolved sodium chloride and potassium nitrate contains sodium, potassium, chloride and nitrate ions. The most common ions in natural waters are:
sodium (Na+) chloride(Cl-) sulfate (SO42-)
calcium (Ca+) boron (H3BO3)
magnesium (Mg+) bicarbonate (HCO3-)
Different waters can have very different proportions of these ions and these proportions can change with time. Some typical analyses of City of San Buenaventura water can be seen in the following chart (2015 Annual Report of the City of San Buenaventura).
Ionic composition of some wells in Ventura
|
Sample |
Na+ |
Ca+ |
Mg+ |
Cl- |
SO42- |
TDS |
EC |
|
|
|
|
(mg/l) |
|
|
|
(umhos/cm) |
|
1 |
200 |
259 |
70 |
92 |
839 |
1668 |
1990 |
|
2 |
45 |
92 |
191 |
44 |
210 |
645 |
874 |
|
3 |
28 |
59 |
21 |
20 |
140 |
316 |
580 |
Total dissolved solids (TDS) and electrical conductivity (EC) are two different ways of measuring the total amount of salts in water. The old way of taking a specified volume (l for liter) of water and boiling it down to the residue which is weighed (mg for milligram) gives TDS. The more modern technique is to measure the electrical current a water will carry (umhos/cm or micromhos/cm), which is in proportion to the number of ions in the water.
Natural waters also contain low concentrations of many other elements. For most, the amounts are too low to be either harmful or beneficial to plants. The main exception is boron which can be a problem for sensitive plants, such as citrus and avocado and probably for cherimoya as well, when in excess of 1 mg/l. Many well waters in Santa Barbara and Ventura Counties contain potentially harmful levels of boron for plants. This is not as common a problem in San Diego County.
In addition to the ions mentioned, there are also those that come from fertilizers and the soil. The main extra ions are potassium, ammonium, nitrate and phosphate. The concentrations of these will depend on the type of soil and the amounts and kinds of fertilizers applied, minus the amounts taken out by plants, held by the soil and lost by leaching or erosion.
In evaluating a water for its potential to harm plants, it is necessary to look at total salinity, as well as the specific ions. Waters with a TDS in excess of 1000 mg/l or an EC greater than 1500 umhos/cm might pose problems for sensitive subtropical plants, and none at all to tolerant plants like figs, apricots or pomegranates. Waters with an excess of sodium and/or chloride (more than 100 mg/l) can induce symptoms that are similar to high levels of salinity.
In most cases, plants respond by initially having their leaf margins turn yellow and die. This happens first on older leaves because they have had the longest time to accumulate the ions. Annual plants are often less affected than perennials, since they do not grow long enough to accumulate sufficient ions to cause damage.
As trees remove water from the soil, the concentration of salts in the remaining soil water increases. Plants adapt to moderate increases, but if the plant is sensitive (and most subtropicals are), it will slow growth in response. If the salt increase is small, the growth reduction will be small and acceptable. But if the level of fertilizer use is high, the water quality poor, or the soil has not been properly leached, the increased soil salinity could reduce growth seriously.
The effects of salinity are usually gradual on plants, unless too much fertilizer has been suddenly applied or strong, dry winds causes rapid drying. Also, with some domestic water there is variation in concentration and kinds of salts in the water with time. The 200 mg/l of sodium in water sample 1 on the chart would be a problem if this were what the homeowner continuously received. However, according to city data, this house does get 94 mg/l at times (not on the chart). The better quality water serves to flush out the higher concentration salts. And this is how to practically deal with poorer quality water, occasionally leach the soil with a volume of water in excess of plant need. When there are no leaching rains, we need to be more aware of the potential for salt accumulation in the soil. With proper plant selection and water management even extremely saline waters can be used.
Water Terminology
The ions in water are measured as parts per million (ppm) or milligrams per liter (mg/l), terms which are interchangeable. This is like saying a percent, but instead of the ions' weight per 100 weight of water, it is the ions' weight per million weight of water. The ion concentration also can appear as milliequivalents per liter (meq/l). A milliequivalent is the ppm of that ion divided by its atomic weight per charge.
Example: Ca2+ with atomic weight of 40 and a solution concentration of possibly 200 ppm. Ca2+ has two charges per atom, so it has an atomic weight of 20 per charge. 200 ppm divided by 20 = 10 meq of calcium for a liter of water.
Total Dissolved Solids (TDS): measure of total salts in solution in ppm or mg/L
Electrical Conductivity (EC): similar to TDS but analyzed differently.
Units: deciSiemens/meter(dS/m)=millimhos/centimeter (mmhos/cm)=
1000 micromhos/cm (umhos/cm).
Conversion TDS to EC: 640 ppm=1 dS/m=1000 umhos/cm
Hardness: measure of calcium and magnesium in water expressed as ppm CaCO
pH: measure of how acid or base the solution
Alkalinity: measure of the amount of carbonate and bicarbonate controlling the pH, expressed as ppm CaCO3.
Sodium Adsorption Ratio (SAR): describes the relative sodium hazard of water
SAR= (Na)/((Ca+Mg)/2)1/2, all units in meq/l
There is also an Adjusted SAR which considers the carbonate and bicarbonate present, but does not do much better in predicting plant response.
General Irrigation Quality Guidelines
(U.C. Leaflet 2995, 1979)
Measurement No problem Increasing Unsuitable
Effect on plant growth
EC (dS/m) 3
Na+ (SAR) 9
Cl- (ppm) 140 140-350 >350
H3BO3 (ppm) 2
Effect on soil permeability
EC (dS/m) >0.5
SAR 9
1.5 feet of water with EC of 1.6 dS/m adds 10,000 # of salt per acre
WATER NEEDS TO BE APPLIED NOT ONLY FOR THE PLANT NEED,
BUT ALSO TO LEACH THE SALTS


- Author: Ben Faber
Thanks for the rains that leach the soils of accumulated salts and bring on new fresh growth. Or maybe not. When we apply irrigation water with salts which with few exceptions we do in irrigated agriculture, salts accumulate in the soil. They accumulate in a certain pattern depending on the type of irrigation and soil type. There's a strong tendency for drip and microsprinklers to form a pattern of salt accumulation near the margins of the wetted patterns. This pattern is stronger with drip because the source point is always pushing a front outward from the emission point. This pattern occurs with microsprinklers, as well, although not as strongly. These patterns continue to form and accumulate as long as there is no rainfall to evenly push the salt down below the root zone. The longer the period of no rain, the larger the salt concentration at the margin.
So the way water moves is generally down. It moves in a wetting front drawn by gravity. It moves laterally too, because of the attraction water has for the soil particles. It will move laterally more in a clay soil than in a sandy soil because there are more particles in a clay soil than a sand (actually more surfaces that hold water). It also carries salt with it. Wherever the water moves, the salt moves. The more rain, the more salt is moved down. The more rain, the deeper the salt is pushed.
The problem with rain, is that if there is not enough, the salt tends to move laterally. In this wet soil solution, the salt is moving from where it is concentrated, to where there is a lower one. And if there isn't enough rain to move that salt down, it just moves back along the salt gradient, back to where the water first came from…….towards the roots. And that salt may be at such a high concentration that it can cause plant damage.
We talk about effective rainfall. This is usually about a quarter of an inch of rain. This is the amount of water to do more than just wet the dust, it's the amount to move water into the root zone. It is also moving salts into the root zone which can be a real problem. A good rain will do more than wet the dust, it will also move the salts out of harm's way in the root zone. The amount of rain necessary to do this going to depend on the salt accumulated and the soil texture. The more salt, the more rain needed. The finer the texture, the more rain. So there is no good cookbook, other than you need enough. And the first rains of the year, watch out. This is often when the highest salt accumulation and the most irregular the rains. Small amounts that can move salt into the root zone.
If there is not enough rain……………The solution !!!!!!!! Run the water to make sure there is enough to move that salt down. Crazy, but a few months ago we had just this situation. It was one of the last rains in the winter and it was not enough to move salts down, and within a week many avocados showed leaf damage. It was sad since we had all been wanting rain, and we wanted a good drenching.
So why am I bringing this up now? Well, the other night I woke up to rain, glorious rain. I enjoyed listening to it and then it stopped. I thought O NO, it's not enough. There are going to be problems. Well luckily most places didn't get and where it did, it was a dust settler. But it made me aware that with the first rains we might see this fall, growers should be on their guard.
Get ready to irrigate with the first rains if they are insufficient for adequate leaching.
![S-AV-CULT-IR[1] S-AV-CULT-IR[1]](https://ucanr.edu/blogs/Topics/blogfiles/39712.jpg)
- Author: Ben Faber
You come on a leaf with the margins munched on. It's got to be a beetle or a looper or some insect doing the damage, right? Not necessarily. It's not time to drag out the Raid. Look at the damage closely. In the photos below you can see the dead leaf margins caused by either salt damage or more likely leaf blight. Leaf blight is a disease that shows up with water stress and is caused by a fungus, one of the Botryoshpaerias. It causes an uneven marginal necrosis that goes along the margin in a somewhat irregular pattern and often not at the leaf tip. In this case it does affect the leaf tip, and since salt burn and leaf blight are caused by the same conditions of water stress, it's probably a bit of both.
Lepidopteran larvae will more commonly feed in a smooth pattern, not the rough pattern seen here. Now with this dead tissue, the wind blows it out, and what's left is the uneven margin. No it's not time to spray an insecticide. It's time to reflect on irrigation. There's a lot of this damage out there now. On avocados, citrus, landscape plants. It's going away until the leaves drop and are replaced with new ones, that will hopefully be well hydrated by rain and proper irrigation.
Top photo is salt/leaf blight damage
Bottom is necrotic tissue that the wind has blown out