- Author: Surendra K. Dara
- Author: Carson DiCicco, Vina Quest
The western grapeleaf skeletonizer (WGLS), Harrisina metallica, is a pest of vineyards in some parts of California. Larval feeding skeletonizes grape leaves and uncontrolled populations can lead to a complete loss of foliage, fruit damage, and yield reduction. WGLS populations are usually suppressed with standard pest management practices used against it or other pests. However, considering regular WGLS infestations in the past few years especially in organic vineyards in warmer parts of the state warrant development of a good monitoring and integrated pest management strategy to improve the pest control efficacy and to minimize the risk of resistance development from potential overuse of limited organic pesticides. An earlier bioassay with biologicals showed azadirachtin, spinosad, Bacillus thuringiensis subsp. aizawai, and entomopathogenic fungi Beauveria bassiana and Metarhizium sp. as potential control options (Dara et al., 2019). The potential of Harrisina brillians granulovirus, a naturally occurring virus that previously suppressed WGLS populations a few decades ago is also explored as a natural solution (Federici and Stern, 1990).
WGLS has 2-3 generations per year with late spring-early summer and mid-late summer infestations in the coastal regions (Fig. 1). Based on the detection of shiny black moths and growing degree-day calculations, pesticide applications can be timed to target hatching larvae. Growing degree-day calculations were made using a model provided by Pest Prophet and temperature data from GreenCast. Good monitoring tools such as traps equipped with lures can be useful to improve the monitoring accuracy especially when the adult activity spreads over multiple weeks for each generation. A study was conducted to assist with the development of new lures for WGLS.
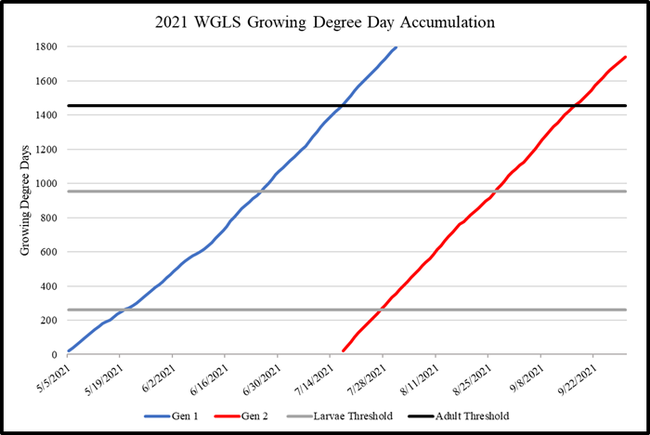
Fig. 1. Growing degree-days indicating WGLS sping and summer generations and thresholds for larvae and adults
Methodology
An organic Cabernet Sauvignon vineyard (San Juan North) in Shandon was used for the study conducted between May and July 2021. Treatments included a blank lure, Pherocon WGLS, TRE 2500, and TRE 2501. The last two are developmental formulations. The pheromone components of the lures are different combinations of 2S-butyl Z7-tetradecenoate, 2-butyl decanoate, 2-butyl dodecanoate, and isopropyl Z7-tetradecenoate to attract male moths and the latter two are new combinations of active ingredients. Each treatment was replicated six times in a randomized complete block design. Within each treatment, a lure was placed in Pherocon VI Delta trap with an adhesive, replaceable liner and tied in the top part of the canopy. A 30 m distance was maintained between the traps with and between replications. Traps were first set up with new lures and liners on 8 May 2021. Adhesive liners were observed every week between 15 May and 3 July 2021 on eight observation dates to count the number of moths. Lures were replaced on 5 June 2021 and adhesive liners were replaced every week or every other week as needed. Data were analyzed using Statistix software and Tukey's HSD test was used to separate significant means.

Pheromone infused lure surrounded by the western grapeleaf sekeltonizer male moths (Above photo by Surendra Dara and below photo by Carson DiCicco)
Results
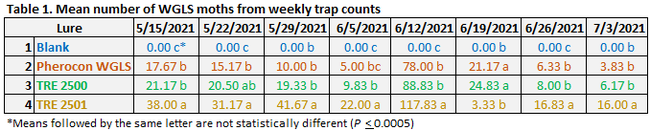
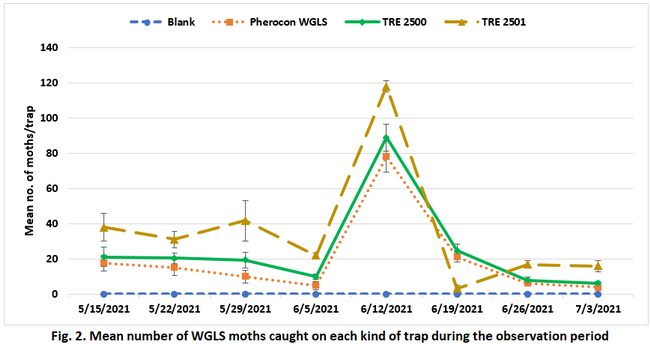
Moth counts significantly (P < 0.0005) varied among the lures on all observation dates (Table 1 and Fig. 2). In general, TRE 2501 lure attracted significantly higher number of moths for most of the observation period. Due to a logistics issue, adhesive liners were not replaced after the moth counts were made on 12 June and those numbers were detected from the next count to derive 19 June moth counts. A lack of space on the liner was probably the reason for having lower moth numbers on 19 June 2021 in TRE 2501. Pherocon WGLS, which is commercially available in the market, was generally less attractive than the developmental formulations. Pheromone combination in the TRE 2501 can be considered for the new formulation for improved monitoring efficacy. Compared to visual monitoring of moth activity, using lures appeared to be an effective strategy for monitoring WGLS, which helped the grower to make effective treatment decisions. On average, 287 moths were captured per each TRE 2501 lure during the 8-week observation period. Considering that each moth can deposit 300 eggs in its lifetime, trapping adults during monitoring can also contribute to reduction in their offspring. In addition to serving as a monitoring tool, lures can also be a control option, if economical.
Acknowledgments: Thanks to Trece for providing lures and traps for the study.
References
Dara, S. K., S. S. Dara, and S. Jaronski. 2019. Biorational control options for the western grapeleaf skeletonizer, a re-emerging pest in California. eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=29081
Federici, B. A. and V. M. Stern. 1990. Replication and occlusion of a granulosis virus in larval and adult midgut epithelium of the western grapeleaf skeletonizer, Harrisina brillians. J. Invertebr. Pathol. 56: 401-414.
- Author: Surendra K. Dara
- Author: Dave Peck, Manzanita Berry Farms

Botrytis cinerea infection appears as wilted flowers and a layer of spores on ripe fruit. Photo by Surendra Dara
Botrytis fruit rot or gray mold caused by Botrytis cinerea is an important disease of strawberry and other crops damaging flowers and fruits. Pathogen survives in the plant debris and soil and can be present in the plant tissues before flowers form. Infection is common on developing or ripe fruits as brown lesions. Lesions typically appear under the calyxes but can be seen on other areas of the fruit. As the disease progresses, a layer of gray spores forms on the infected surface. Severe infection in flowers results in the failure of fruit development. Cool and moist conditions favor botrytis fruit rot development. Sprinkler irrigation, rains, or certain agricultural practices can contribute to the dispersal of fungal spores.
Although removal of infected plant material and debris can reduce the source of inoculum in the field, regular fungicide applications are typically necessary for managing botrytis fruit rot. Since fruiting occurs continuously for several months and fungicides are regularly applied, botrytis resistance to fungicides is not uncommon. Applying fungicides only when necessary, avoiding continuous use of fungicides from the same mode of action group (check FRAC mode of action groups), exploring the potential of biological fungicides to reduce the risk of resistance development are some of the strategies for effective botrytis fruit rot management. In addition to several synthetic fungicides, several biological fungicides continue to be introduced into the market offering various options for the growers. Earlier field studies evaluated the potential of various biological fungicides and strategies for using them with synthetic fungicides against botrytis and other fruit rots in strawberry (Dara, 2019; Dara, 2020). This study was conducted to evaluate some new and soon to be released fungicides in fall-planted strawberry to support the growers, ag input industry, and to promote sustainable disease management through biological and synthetic pesticides.
Methodology
This study was conducted at the Manzanita Berry Farms, Santa Maria in strawberry variety 3024 planted in October, 2020. While Captan and Switch were used as synthetic standards, a variety of biological fungicides of microbial, botanical, and animal sources were included at various rates and different combinations and rotations. Products and active ingredients evaluated in this study included Captan Gold 4L (captan) from Adama, Switch 62.5 WG (cyprodinil 37.5% + fludioxinil 25%) from Syngenta, NSTKI-14 (potassium carbonate 58.04% + thyme oil 1.75%) from NovoSource, A22613 [A] (botanical extract) from Syngenta, Regalia (giant knotweed extract 5%) from Marrone Bio Innovations, EXP14 (protein 15-20%) from Biotalys, Gargoil (cinnamon oil 15% + garlic oil 20%) and Dart (caprylic acid 41.7% + capric acid 28.3%) from Westbridge, Howler (Pseudomonas chlororaphis strain AFS009), Theia (Bacillus subtilis strain AFS032321), and Esendo (P. chlororaphis strain AFS009 44.5% + azoxystrobin 5.75%) from AgBiome, ProBlad Verde (Banda de Lupinus albus doce – BLAD, a polypeptide from sweet lupine) from Sym-Agro with Kiplant VS-04 (chitosan 2.3%) or Nu-Film-P spreader/sticker, AS-EXP Thyme (thyme oil) from AgroSpheres, and AgriCell FunThyme (thyme oil) provided by AgroSpheres.
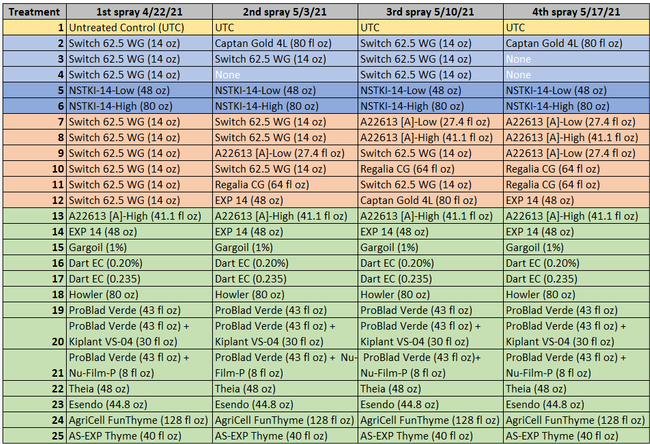
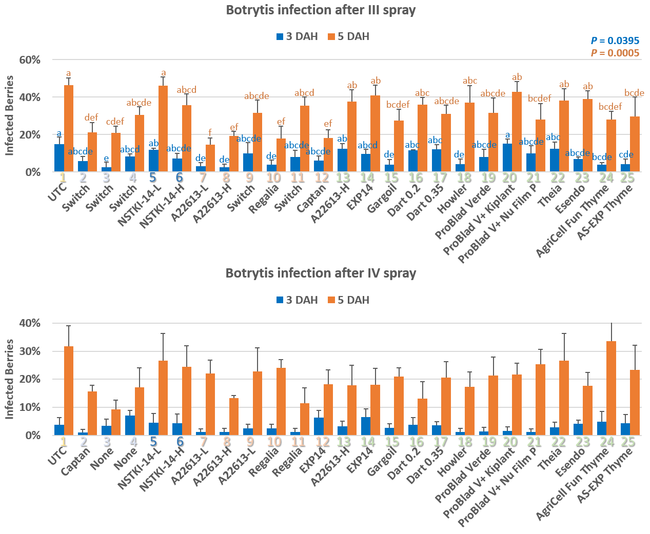
Table 1. List of treatments color coded according to the kind of fungicide (light blue=synthetic fungicide; dark blue=synthetic+biological fungicide active ingredient; peach=synthetic and biological fungicides alternated; green=biological fungicides)
Excluding the untreated control, rest of the 24 treatments can be divided into synthetic fungicides, a fungicide with synthetic + biological active ingredients (a formulation with two application rates), synthetic fungicides alternated with biological fungicides, and various kinds of biological fungicides (Table 1). Treatments were applied at a 7-10 day interval between 22 April and 17 May, 2021. Berries for pre-treatment disease evaluation were harvested on 19 April, 2021. Each treatment had a 5.67'X15' plot replicated four times in a randomized complete block design. Strawberries were harvested 3 days before the first treatment and 3-4 days after each treatment for disease evaluation. On each sampling date, marketable-quality berries were harvested from random plants within each plot during a 30-sec period and incubated in paper bags at outdoor temperatures under shade. Number of berries with botrytis infection were counted on 3 and 5 days after harvest (DAH) and percent infection was calculated. This is a different protocol than previous years' studies where disease rating was made on a 0 to 4 scale. Treatments were applied with a backpack sprayer equipped with Teejet Conejet TXVK-6 nozzle using 90 gpa spray volume at 45 PSI. Water was sprayed in the untreated control plots. Dyne-Amic surfactant at 0.125% was used for treatments that contained Howler, Theia, Esendo, AgriCell FunThyme, AS-EXP Thyme, and EXP 14. Research authorization was obtained for some products and crop destruction was implemented for products that did not have California registration.
Percent infection data were arcsine-transformed before subjecting to the analysis of variance using Statistix software. Significant means were separated using the least significant difference test.
Results
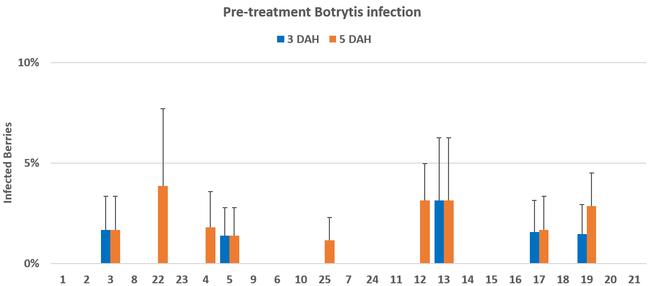
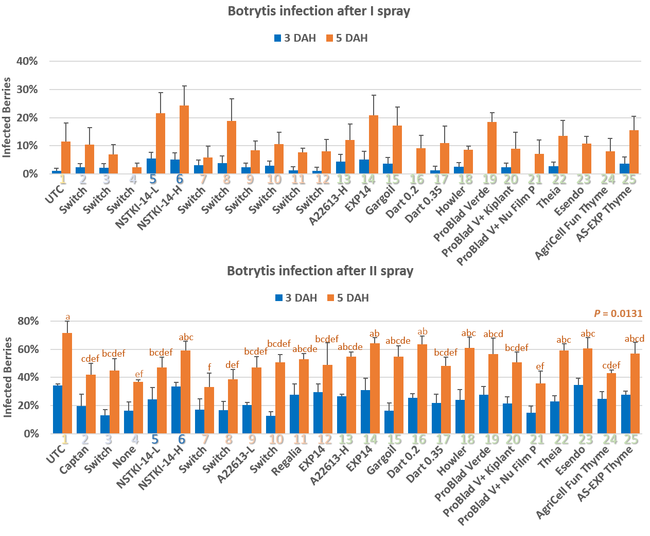
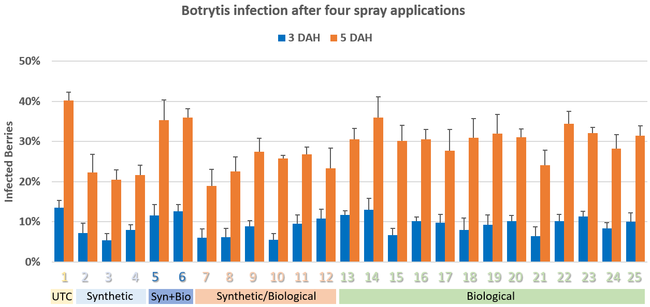
Pre-treatment infection was very low and occurred only in some treatments with no statistical difference (P > 0.05). Infection levels increased for the rest of the study period. There was no statistically significant difference (P > 0.05) among treatments for disease levels 3 or 5 days after the first spray application. Differences were significant (P = 0.0131) in disease 5 DAH after the second spray application where 13 treatments from all categories had significantly lower infection than the untreated control. After the third spray application, infection levels were significantly lower in eight treatments in 3 DAH observations (P = 0.0395) and 10 treatments in 5 DAH observations (P = 0.0005) compared to untreated control. There were no statistical differences (P > 0.05) among treatments for observations after fourth spray application or for the average of four applications. However, there were numerical differences where infection levels were lower in several treatments than the untreated control plots.
In general, the efficacy of both synthetic and biological fungicides varied throughout the study period among the treatments. When the average for post-treatment observations was considered, infection was numerically lower in all treatments regardless of the fungicide category. Multiple biological fungicide treatments either alone or in rotation with synthetic fungicides appeared to be as effective as synthetic fungicides.
Conclusions
Botanical and microbial fungicides can be effective against either for using alone or in rotation with synthetic fungicides for suppressing botrytis fruit rot in strawberry. Additional studies can help optimize the application rates and use strategies for those fungicides that were not as effective as others. Sanitation practices and use of synthetic and biological fungicides help manage botrytis fruit rot.
Acknowledgements: Thanks to AgBiome, AgroSpheres, Biotalys, NovaSource, Sym-Agro, Syngenta, and Westbridge for funding and Chris Martinez for his technical assistance.
References
Dara, S. K. 2019. Five shades of gray mold control in strawberry: evaluating chemical, organic oil, botanical, bacterial, and fungal active ingredients. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=30729
Dara, S. K. 2020. Evaluating biological fungicides against botrytis and other fruit rots in strawberry. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=43633
- Author: Surendra K. Dara
Biopesticides contain active ingredients of natural or biological origin that include plant extracts, microorganisms, microbial metabolites, organic molecules, minerals, or other such natural materials that have pesticidal properties. Pests such as herbivorous arthropods, pathogens, parasitic nematodes, mollusks, rodents, and weeds cause significant crop damage when they are not managed. Pest suppression is a critical part of crop production to maintain plant health, prevent yield losses, and optimize returns. As agriculture advanced from subsistence farming to a global enterprise, crop protection also evolved over millennia. When farming was less organized, nature maintained a balance and provided solutions initially. Then natural solutions were actively implemented until industrialization led to the use of synthetic inputs in the 20th century. While synthetic fertilizers and pesticides contributed to a tremendous improvement in the yield potential, the indiscriminate use of some of them and the resulting damage to the environment and human health steered food production in the recent past towards organic farming with the use of nature-based solutions.
Although biopesticides have been around for a few decades, the growth of organic farming gave an impetus to the biopesticide industry during the past few years resulting in the development of new active ingredients and improved formulations. Now, biopesticides are considered an important part of integrated pest management (IPM) strategies in both organic and conventional systems. With a considerable industry investment in research and development, the quality and efficacy of biopesticides have also significantly improved. This has also contributed to optimizing the cost of some formulations. However, there is still a need to fill the knowledge gaps in biopesticides and their use. Depending on the active ingredient, the mode of action for biopesticides, their target pests, their storage and handling, and the use strategies are quite diverse, and a thorough understanding of these aspects is critical for their successful use. As emphasized in the new IPM model (Dara, 2019), while biopesticide use is an integral part of crop protection, understanding the pest biology, using biopesticides appropriate for the target life stage of the pest, applying them at the right time and rate using the right technology, avoiding incompatibility issues, building and sharing effective use strategies, and continuously investing in research and outreach are essential elements of biopesticide use. Biopesticides also play an important role in insecticide resistance management (IRM) to address resistance issues associated with synthetic pesticides. This article provides an overview of various biopesticide categories and general strategies for their successful use for IPM and IRM.
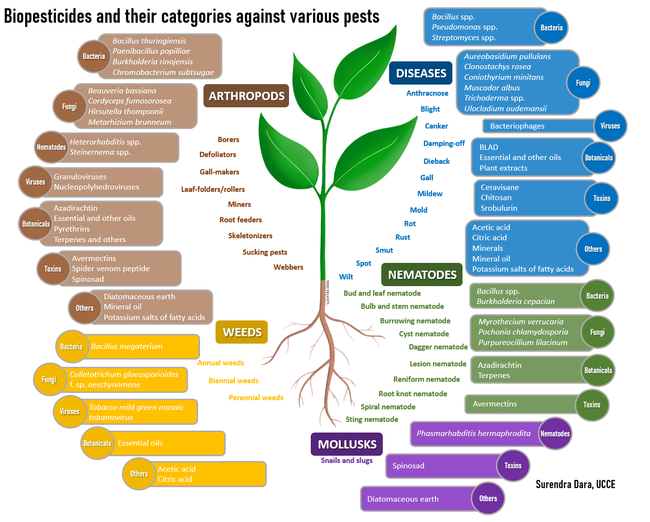
Biopesticides can be used for managing arthropod pests, bacterial or fungal pathogens, plant-parasitic nematodes, weeds, and snails and slugs. Some formulations or active ingredients have multiple roles and can be effective against more than one category of pests. While some active ingredients are very specific to a particular pest or related species, others have a broad-spectrum activity. Based on the source, biopesticides can be placed in four broad categories: i) botanicals, ii) microbials, iii) toxins, and iv) minerals and other natural materials.
Botanical extracts: Plants are a rich source of numerous phytochemicals or secondary metabolites that have a wide range of properties including pesticidal activity. Acids, alkaloids, flavonoids, glycosides, saponins, and terpenoids in plant extracts or oils obtained from seeds and other plant parts are some of the compounds present in various biopesticides (Pino et al., 2013). Azadirachtin, BLAD (polypeptide from sweet lupine seeds), citric acid, essential oils, pyrethrins, soybean oil, and extract of the giant knotweed are used for their acaricidal, insecticidal, fungicidal, nematicidal, or herbicidal properties.
Microbials: Some of the microbial pesticides have live microorganisms (such as entomopathogens, Bacillus spp., Streptomyces spp., and Trichoderma spp.) while others (such as Burkholderia rinojensis and Chromobacterium subtsugae)have heat-killed microorganisms and fermentation solids as the active ingredients. Entomopathogenic microorganisms [Bacillus thuringiensis (bacterium), Beauveria bassiana and Cordyceps fumosorosea (fungi), Heterorhabditis spp. and Steinernema spp. (nematodes), and granuloviruses and nucleopolyhedroviruses] primarily kill their hosts through infection; microbe-based fungicides antagonize plant pathogens through competitive displacement and production of toxic metabolites; nematophagous fungi parasitize plant-parasitic nematodes; and plant pathogenic bacteria, fungi, and viruses infect and suppress weeds. Bacteriophages, which are viruses that parasitize bacteria, are used against the plant pathogenic species of Clavibacter, Erwinia, Pseudomonas, Xanthomonas, Xylella, and other genera.
Toxins and other organic molecules: There are multiple examples of toxic organic molecules derived from various organisms. Avermectins from the bacterium Streptomyces avermitilis and spinosad from the bacterium Saccharopolyspora spinosa, strobilurin from the mushroom Strobuluris tenacellus, and cerevisane from the yeast Saccharomyces cerevisae are some of the microbial toxins that are effective against insects, plant-parasitic nematodes, or snails and slugs. A venom peptide from the Blue Mountains funnel-web spider, Hadronyche versuta, from Australia is a recently developed insecticide active ingredient with its unique mode of action class. Chitosan, a polysaccharide from the exoskeleton of shellfish, is a fungicide.
Minerals and other natural materials: Diatomaceous earth, mineral oil, and minerals such as sulfur are used for controlling multiple categories of pests. Potassium salts of fatty acids of plant or animal origin, known as insecticidal soap, have insecticidal and fungicidal properties. Organic acids such as acetic acid and citric acid are derived from plants and have fungicidal and herbicidal properties. Since these are different from other botanical extracts, they are placed in this category.
Except for the microbial pesticides that have live microorganisms, most biopesticides have chemical molecules of microbial, fungal, botanical, or mineral origin and work through various modes of action similar to synthetic pesticides. Several synthetic pesticides are developed from natural molecules. Abamectin, pyrethroids, neonicotinoids, spinetoram, and storbulurins are synthetic analogs based on avermectins, pyrethrins, nicotine, spinosad, and strobulurin, respectively, and were developed for improved stability, safety, or ease of commercial-scale production.
Integrated pest management and resistance management: Biopesticides are very diverse in their origin and mode of action and have been successfully used in several cropping systems for managing a variety of pests. They have complex interactions with plants, soil microbiota, pests, and environmental conditions. It is critical to have a good understanding of the source of biopesticides and how they act on their target pests. Certain biopesticides may have special storage and handling requirements or tank-mixing restrictions. It is essential to refer to the manufacturer's guidelines or label instructions to avoid incompatible tank-mix combinations, understand proper application sequences, and to store, transport, and apply under unfavorable conditions. While it is very important to use biopesticides as a part of the IPM program and tools for IRM, caution is warranted to avoid repeated use of the same or a similar type of biopesticide. Pests can develop resistance to biopesticides just as they do to synthetic pesticides (Dara, 2020).
Strategies for using biopesticides: From the seed or transplant treatment to soil or foliar application, biopesticides can be used throughout crop production. Certain combinations can have an additive or a synergistic effect on pest suppression. At the same time, certain inputs or practices can negatively impact biopesticide efficacy. For example, alkaline tank-mix components breakdown the protein coat of entomopathogenic viruses and Bacillus thuringiensis. Botanical oils can be incompatible with cold water. Some fungicides such as captan and thiram are incompatible with entomopathogenic fungi like Beauveria bassiana while several others are compatible (Dara et al., 2014).
Investing in biopesticides: Environmental safety and resistance development are two major concerns for excessive use of synthetic pesticides and incorporating biopesticides into IPM will help address both issues. Substituting biopesticides for synthetic pesticides will reduce the total amount of the latter during a production season and their potential negative impact on the environment and human health. Several biopesticides are not harmful to pollinators and in some production systems, pollinators are used to deliver biopesticides to the crops they pollinate. Adding biopesticides to the standard crop protection program will also increase pest control efficacy. Additionally, by not continuously using synthetic pesticides, the risk of resistance will be reduced and thus their efficacy will continue to be maintained. Although some biopesticides can be more expensive than synthetic pesticides, investing in them will be a good strategy for both the short-term benefit of effective pest suppression and the long-term benefit of a healthy and resilient ecosystem. Since pests do not have boundaries, area-wide implementation of good agricultural practices with a balanced use of synthetic and natural inputs is necessary for maintaining the productivity of the cropping systems.
Productive collaborations among the pesticide industry, researchers, extension educators, and the grower community are critical for successfully using biopesticides for sustainable food production. While research helps to develop effective formulations and their use strategies, outreach helps with the implementation of those strategies.
References
Dara, S.S.R., S. S. Dara, A. Sahoo, H. Bellam, and S. K. Dara. 2014. Can entomopathogenic fungus Beauveria bassiana be used for pest management when fungicides are used for disease management? UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=15671
Dara, S. K. 2019. The new integrated pest management paradigm for the modern age. J. Integr. Pest Manag. 10 (1): 12. https://doi.org/10.1093/jipm/pmz010
Dara, S. K. 2020. Arthropod resistance to biopesticides. Organic Farmer 3 (4): 16-19. https://organicfarmermag.com/2020/08/arthropod-resistance-to-biopesticides/
Pino, O. Y. Sánchez, and M. M. Rojas. 2013. Plant secondary metabolites as an alternative in pest management. I: Background, research approaches and trends. Rev. ProtecciónVeg. 28 (2): 81-94.
- Author: Surendra K. Dara
The diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is an important pest of broccoli, Brussels sprouts, cabbage, cauliflower, collards, kale, and other cruciferous crops. It exclusively feeds on cultivated and weedy crucifers and has a worldwide distribution. Larvae feed on foliage and growing parts of young plants or bore into the heads or flower buds resulting in skeletonization of leaves, stunting of the plants, or failure of head formation in some hosts. In warmer areas, the diamondback moth has up to 12 generations per year. While multiple species of parasitoids and predatory arthropods provide some level of natural control, insecticidal applications are a primary means of diamondback moth management. Although several synthetic and biological pesticides are effective against the diamondback moth, resistance to Bacillus thuringiensis (Ferré et al. 1991), abamectin (Pu et al. 2009), emamectin benzoate, indoxacarb, and spinosad (Zhao et al. 2006), pyrethroids and other pesticides (Leibee and Savage 1992; Endersby et al. 2011) has been well-known from around the world. Excessive use of any kind of pesticide leads to resistance problems (Dara 2020; also see a video presentation) to an individual pesticide or multiple pesticides. Integrated pest management (IPM) strategy encourages the use of various control options both for maintaining pest control efficacy and reducing the risk of resistance development (Dara 2019). Regularly monitoring the pest populations to make treatment decisions, rotating pesticides with different modes of action, exploring the potential of biocontrol agents, and other non-chemical control approaches such as mating disruption with pheromones are some of the IPM strategies for controlling the diamondback moth. While sex pheromones effectively used to manage several lepidopteran pests and are proven to be a critical IPM tool, mating disruption is not fully explored for controlling the diamondback moth. A study was conducted in Brussels sprouts to evaluate the efficacy of a sprayable pheromone against the diamondback moth and to enhance the current IPM strategies.
Methodology
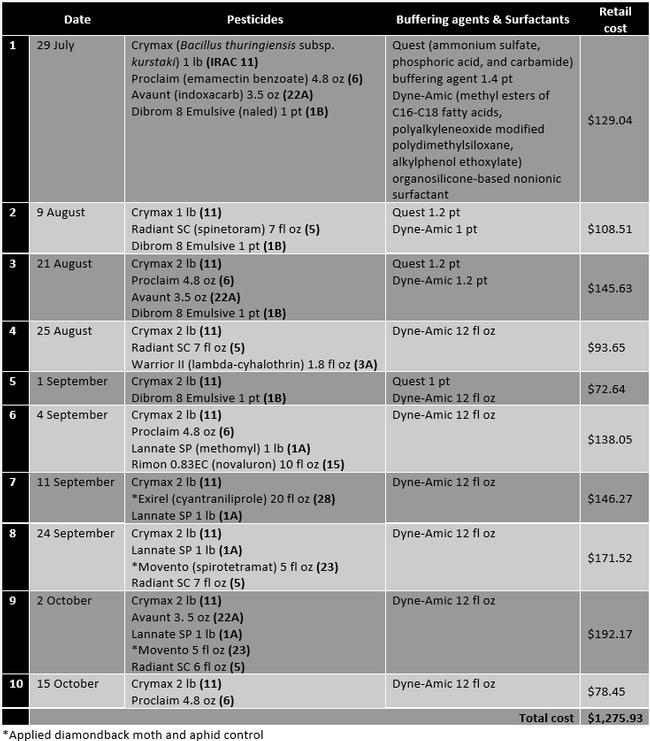
The study was conducted on a 10-acre Brussels sprouts field in Santa Maria. Cultivar Marte was planted in early July 2020 with expected harvesting in mid to late December. A typical diamondback control program includes monitoring diamondback moth populations with the help of sticky traps and lures and applying various combinations of biological and synthetic pesticides at regular intervals. This study evaluated the efficacy of adding CheckMate DBM-F to the grower standard practice of monitoring the diamondback moth populations with traps and lures and applying pesticides. Treatments included i) grower standard pesticide program (Table 1) and ii) grower standard pesticide program with two applications of 3.1 fl oz of CheckMate DBM-F on 9 August and 11 September. Treatment materials were applied by a tractor-mounted sprayer using a 100 gpa spray volume and necessary buffering agents and surfactants. Each treatment was 5 ac and divided into four quadrants representing four replications. In the middle of each quadrant, one Suterra Wing Trap was set up with a Trécé Pherocon Diamondback Moth Lure. Lures were replaced once a month in early September and early October. Sticky liners of the traps were replaced every week to count the number of moths trapped. Traps were placed on 1, 12, and 24 August, 1, 11, 18, and 27 September, and 6 October and the moth counts were taken from respective traps on 8 and 20 August, 1, 11, 18, and 27 September, 6 and 15 October. CheckMate DBM-F was applied at 3.1 fl oz/ac on 9 August and 11 September. The number of larvae and their feeding damage on a scale of 0 to 4 (where 0=no damage, 1=light damage, 2=moderate damage, 3=high damage, 4=extensive/irrecoverable) were recorded from 25 random plants within each replication on 30 August and 6 and 18 October. Data were subjected to analysis of variance using Statistix software and significant means were separated using Tukey's HSD test. The retail value of various pesticides was also obtained to compare the cost of treatments.
Table 1. Pesticides, buffering agents, and surfactants, their active ingredients, rates/ac (along with the IRAC mode of action groups), and retail pricing for those applied in the grower standard diamondback moth control program.
When CheckMate DBM-F [(Z)-11-Hexadecenal (3) , (Z) - 11 - Hexadecen-1-yl Acetate (1)] was applied the first time on 9 August, Dibrom 8 Emulsive was replaced with Warrior II, the buffering agent Quest was not used, and the surfactant Dyne-Amic was replaced with Induce (dimethylpolysiloxane) to avoid potential compatibility issues. The impact of this substitution is expected to be negligible within the scope of this study. The retail cost of 3.1 fl oz CheckMate DBM-F is $45.60. The cost of lures and traps would be about $4-8 per acre for a six-month crop like Brussels sprouts.
Results and Discussion
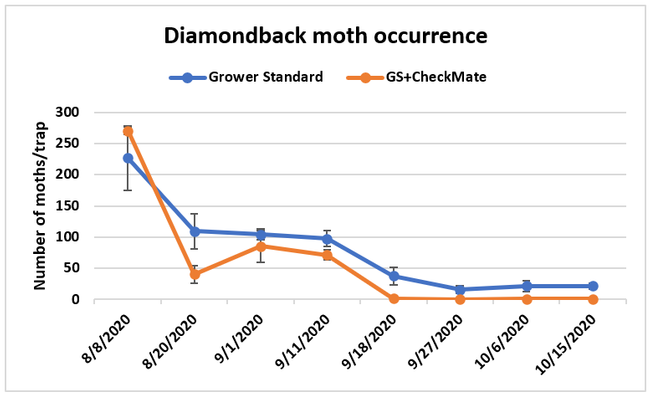
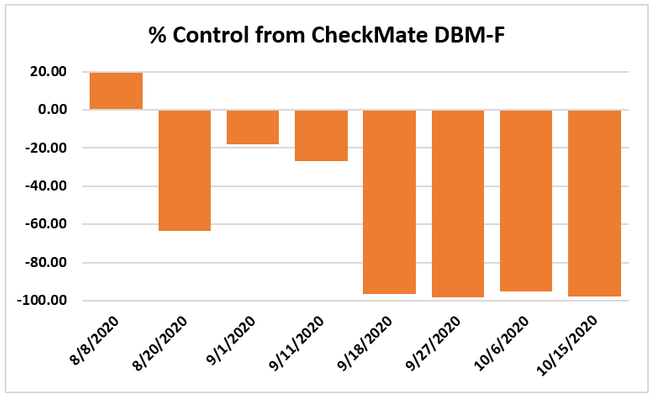
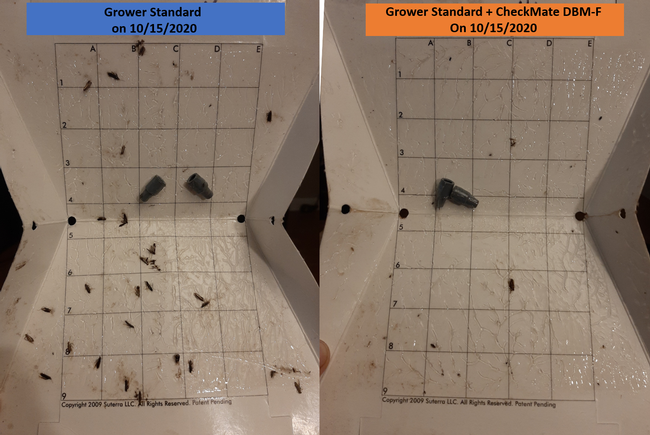
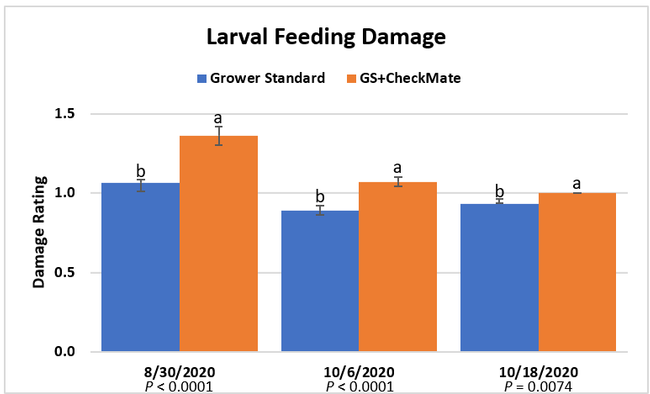
Moth populations: Traps in replication 4 in both treatments on 8 August and replication 1 in the grower standard were missing, probably knocked down by a tractor. The day before CheckMate DBM-F was first applied, the mean number of adult diamondback moths caught were 227 in the grower standard and 271 in the plots that would receive the pheromone application. There was a gradual decline in moth counts during the rest of the observation period in both treatments. However, the decline was higher in the plots that received CheckMate DBM-F. The number of moths per trap were about 19% higher in the pheromone-treated plots compared to the grower standard before the study but were nearly 98% lower by the end of the study. The reduction in moth populations from mating disruption was significant on 18 September (P =0.039) and 15 October (P = 0.006).
Larval populations: The mean number of larvae per 25 plants in a replication was zero on all observation dates except for 0.01 on 30 August in the plots that received CheckMate. Four insecticide applications by the time the study was initiated, and the remaining six applications effectively suppressed larval populations.
Damage ratings: Larval feeding damage ratings were consistently low (P < 0.0001) in the plants that did not receive CheckMate DBM-F. The damage was limited to the older leaves at the bottom of the plants and must have been from early feeding before the initiation of the study. The lack of larvae and the evidence of new feeding damage also confirm that the crop remained healthy and pest-free.
Since frequent pesticide applications effectively suppressed larval populations and prevented their feeding damage, the effectiveness of mating disruption in reducing yield losses could not be determined in this study. Since larval counts were not made weekly or between pesticide applications, those that were probably present between the pesticide applications could not be determined. Moths found in the traps probably developed from the larvae in the field or could have been those that flew in from other areas. However, lower moth populations in CheckMate DBM-F treatment demonstrated the overall influence of mating disruption and pest suppression.
It is common to make about 10-12 pesticide sprays during the 6-month crop cycle of Brussels sprouts. The cost of each application varied from about $73 to $192 depending on the materials used with an average cost of about $128 per application in this study. The cost of two CheckMate DBM-F applications is $91. If diamondback moth populations could be reduced with mating disruption, it is estimated that 2-3 pesticide applications could be eliminated. That results in $164 to $292 of saving for the pesticide costs and additional savings in the application costs per acre by investing $91 in the mating disruption. Since the diamondback moth can develop resistance to several chemical and natural pesticides, eliminating some applications as a result of mating disruption also contributes to resistance management along with potential negative impact of pesticides on the environment. Compared to other mating disruption strategies, a sprayable formulation compatible with other agricultural inputs is easier and cost-effective to use.
This study demonstrated that mating disruption with CheckMate DBM-F will significantly enhance the current IPM practices by reducing pest populations, contributing to insecticide resistance management, and reducing pest management costs. Additional studies, with fewer pesticide applications that allow larvae to survive and cause some damage, might further help understand the role of mating disruption where pest populations are not managed as effectively as in this field.
Watch a presentation of this study
Acknowledgments: Thanks to the PCA and the grower for their research collaboration, Tamas Zold for his technical assistance in data collection, Ingrid Schumann for market research of pesticide pricing, and Suterra for the financial support.
References
Dara, S. K. 2019. The new integrated pest management paradigm for the modern age. J. Int. Pest Manag. 10: 12.
Dara, S. K. 2020. Arthropod resistance to biopesticides. Organic Farmer 3 (4): 16-19.
Endersby, N. M., K. Viduka, S. W. Baxter, J. Saw, D. G. Heckel, and S. W. McKechnie. 2011. Widespread pyrethroid resistance in Australian diamondback moth, Plutella xylostella (L.), is related to multiple mutations in the para sodium channel gene. Bull. Entomol. Res. 101: 393.
Ferré, J., M. D., Real, J. Van Rie, S. Jansens, and M. Peferoen. 1991. Resistance to the Bacillus thuringiensis bioinsecticide in a field population of Plutella xylostella is due to a change in a midgut membrane receptor. Proc. Nat. Acad. Sci. 88: 5119-5123.
Leibee, G. L. and K. E. Savage. 1992. Evaluation of selected insecticides for control of diamondback moth and cabbage looper in cabbage in Central Florida with observations on insecticide resistance in the diamondback moth. Fla. Entomol. 75: 585-591.
Pu, X., Y. Yang, S. Wu, and Y. Wu. 2009. Characterisation of abamectin resistance in a field-evolved multiresistant population of Plutella xylostella. Pest Manag. Sci. 66: 371-378.
Zhao, J-Z., H. L. Collins, Y-X. Li, R.F.L. Mau, G. D. Thompson, M. Hertlein, J. T. Andaloro, R. Boykin, and A. M. Shelton. 2006. Monitoring of diamondback moth (Lepidoptera: Plutellidae) resistance to spinosad, indoxacarb, and emamectin benzoate. J. Econ. Entomol. 99: 176-181.
- Author: Surendra K. Dara
Several crown, fruit, and foliar diseases cause significant yield losses to strawberry. Gray mold or Botrytis fruit rot caused by Botrytis cinerea, mucor fruit rot by Mucor spp., and Rhizopus fruit rot by Rhizopus spp. are common fungal diseases in California. Botrytis cinerea is more prevalent and damaging fungus among these pathogens warranting regular fungicidal applications. Fungal spores survive in plant debris and soil and infection can occur before flower initiation. Both flowers and fruits are subjected to infection. Severely infected flowers fail to develop into fruits. Infection on developing or ripe fruit occurs as brown lesions, usually under calyxes. Infected areas rot and become dry and leathery under dry conditions or produce a thick, gray mat of spores under cool, moist conditions.
Mucor spp. invade the fruit through ruptured skin and cause leaky fruit rot. Under high humidity, profuse fungal growth of white, tough filaments with black spore-bearing structures is seen covering the fruit. In the case of Rhizopus fruit rot, discolored, water-soaked spots develop on fruit eventually leading to wilting. Similar to the Mucor fruit rot, Rhizopus rot also leads to leaky fruits and development of black spore-bearing structures on white mycelia under high humidity. Both pathogens survive in dead and decaying plant material and can persist in the field.
In a fall-planted conventional strawberry, growers usually make 12 or more fungicidal applications during a four-month period to control Botrytis and other fruit rots. Although fungicides with different modes of action are present and growers try to rotate them, fungicide resistance in B. cinerea is common and effective integrated disease strategies are necessary. Using biostimulants that might improve plant's ability to withstand diseases and alternating chemicals with biological fungicides could be some options to mitigate chemical fungicide resistance. Previous studies looked at the response of fruit diseases to various treatments that received biological soil amendments (Dara, 2020a), soil fungicides (Dara, 2020b), or chemical and biological fungicides (Dara, 2019). This study was conducted to evaluate the efficacy of some biological fungicides along with a chemical fungicide primarily against Botrytis fruit rot.
Methodology
This study was conducted at a research strawberry field at the Shafter Research Station. Strawberry cultivar San Andreas was planted on 31 October 2019. Other than regular irrigation and fertigation, plants in this study were not treated with any agricultural inputs for agronomic or pest management purposes. Treatments included i) untreated control, ii) Elevate 50 WDG (fenhexamid) at 8 oz/ac, iii) Serifel (Bacillus amyloliquefaciens) at 8 oz/ac, iv) ProBlad Verde (Banda de Lupinus albus doce – BLAD, a polypeptide from sweet lupine) at 36 fl oz with Cinnerate (cinnamon oil) at 0.25% followed by ProBlad Verde at 36, 43, and 43 fl oz/ac on subsequent applications, and v) ProBlad Verde at 36 fl oz with Cinnerate at 0.25% followed by three subsequent applications of ProBlad Verde at 32 fl oz/ac. Each treatment had a 3.2' wide and 14' long plot with two rows of plants and replicated four times in a randomized complete block design. Treatments were applied using a CO2-pressurized backpack sprayer using a 45 gpa spray volume on 26 March, 2, 10, and 20 April 2020. Flowers and fruits were removed from all the plants before the first application. Fruit was harvested on 14 and 27 April and 2 and 10 May and stored in vented plastic containers for postharvest quality assessment. The severity of Botrytis and other fruit rots was recorded 3 and 5 days after harvest on a scale of 0 to 4 where 0=no disease, 1=1-25% fruit with fungal infection, 2=26-50% infection, 3=51-75%, and 4=76-100%. Compared to Botrytis fruit rot, other rots occurred as mixed infections at different times and it was not possible to accurately measure them separately. Data presented in this study primarily represent Botrytis fruit rot with other fruit rots included on some data sets. Data were subjected to analysis of variance using Statistix software to compare disease severity for individual harvest dates and their average.
Results
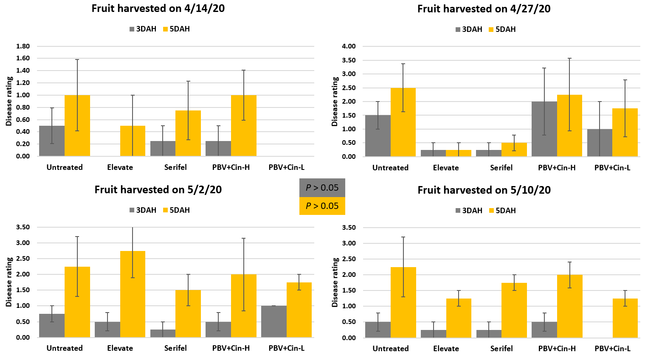
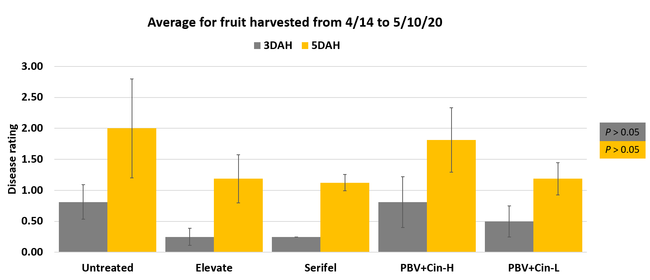
Fruit rots occurred from low to moderate levels during the observation period. Disease severity followed the usual trend with higher levels 5 days after harvest compared to 3 days after harvest. Compared to untreated control, disease severity was numerically lower in some treatments especially 3 days after harvest, but differences were not statistically significant (P > 0.05) when individual harvest dates or their average were considered. The average disease severity from four harvests was 0.25 in Elevate and Serifel, 0.50 in ProBlad Verde low rate with Cinnerate, and 0.81 in ProBlad Verde high rate with Cinnerate treatment and untreated control 3 days after harvest. The average disease severity was 1.13 for Serifel, 1.19 for Elevate and the low rate of ProBlad Verde with Cinnerate, 1.81 for the high rate of ProBlad Verde with Cinnerate, and 2.0 for untreated control 5 days after harvest. Although statistically significant differences could not be found among treatments, this study indicates the potential of non-chemical alternatives and warrants additional studies for further investigation.
Acknowledgements: Thanks to BASF and Sym-Agro for funding this study and Marjan Heidarian Dehkordi and Zach Woolpert for the technical assistance.
References
Dara, S. K. 2019. Five shades of gray mold control in strawberry: evaluating chemical, organic oil, botanical, bacterial, and fungal active ingredients. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=30729
Dara, S. K. 2020a. Improving strawberry yields with biostimulants and nutrient supplements: a 2019-2020 study. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=43631
Dara, S. K. 2020b. Impact of drip application of fungicides on strawberry health and yields. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=43632