
- Author: Mark Bolda
- Author: Michael Cahn
The recent flooding on the Central Coast has really put a lot of local strawberry growers in a bad position. Some fields have been underwater several days to a week or more, which has deprived plants of oxygen and sunlight needed for respiration and growth. As these plants try to recover, they will likely be severely set back for several weeks. Other fields that were briefly flooded are silted over and have completely saturated soils.
Looking beyond our current conditions of just too much water, we need think about what the plan of action is going to be going forward into the spring. Since in many areas strawberry plants have been set back because they were in saturated, anaerobic soil, growers will need to turn their attention to jump starting growth in their fields once the ground dries out, and becomes aerobic again.
It will be important at this time to think about nutrition. It's a fair bet that even fields that received pre-plant fertilizer lost a significant amount of nitrogen from the soil during the flooding and unprecedented rains during the last few months. We could be wrong about this, so the first thing growers and farm managers should do once they are back out in the fields is take a soil sample and check the mineral N levels, especially the concentration of nitrate, which is the form of nitrogen that strawberry readily take up. Nitrate-N will also mineralize from the soil organic matter and any organic amendments that were previously incorporated in the soil as the soil becomes oxygenated again. Soil samples should be collected from 8 to 12 locations in the field from the 0 to 12-inch depth in or near the plant row and composited together. A subsample of the composited soil should be analyzed for mineral forms of nitrogen (nitrate and ammonium). We recommend using the soil nitrate quick test to assess the soil nitrate status of your fields in a timely manner. Please refer to this previous article on the how to accurately measure soil nitrate using the quick test. If you intend to send the soil to a laboratory that can quickly analyze the soil, we suggest shipping the soil sample with blue ice so that it stays cold to prevent mineralization of N in transit. The laboratory should analyze the sample for both ammonium and nitrate, the two mineral forms of nitrogen that are in the soil.
If the soil nitrate values are below 10 ppm nitrate-N then the plants will likely benefit from an addition of N fertilizer. Fertilizers containing nitrate forms of N such as CAN-17, UAN32, or ammonium nitrate would be good to add so that the strawberry plants can immediately take up nitrogen, which should help jump start growth. The urea and ammonium contained in fertilizer will also mineralize to nitrate, but due to the recent anaerobic conditions and cold soil temperatures, this process may be slower than normal.
Since this might be the first time that you've run your irrigation system in a while, it'll be good to check before fertigating to ensure that everything is in good order. Check for broken connections between drip lines and the submains (layflat, oval hose). Check that valves and pipes are still connected and unbroken and that the pump is functioning well. These will be all good items to check before putting this system to work after such a long hiatus.


- Author: Mark Bolda
Below is a look at what happens to a soil following application of mustard seed meal (MSM) at 1.5 T per acre and mustard seed meal (again 1.5 T per acre) + crab meal (500# per acre) as separate treatments two weeks after fumigation with Ally 33 (67% AITC, 33% chloropicrin applied at 340# per acre on Oct 7).
Grower standard was methyl bromide/chloropicrin applied at 350# per acre. Planting took place Nov 3.
A soil sample taken on Nov 7 did not show differences in soil aspects analyzed between any of the treatments, although ammonium - N concentrations were surprisingly high (30 ppm and up) and nitrate - N numbers tended to be quite low (6 ppm and below).
Remarkably, look what has happened in the 4 weeks since that sample. Bear in mind that the grower has since sprinkled overhead several times and we had a good amount of rain as well. Commenting continues below the tables.
Unless otherwise indicated, units are in ppm of dry soil.
Table 1A. Soil analysis from December 7, 2016
|
Sample |
pH |
EC (dS/m) |
Nitrate – N |
Ammonium – N |
|
Methyl bromide grower standard |
7.4 |
0.9 |
11.3 |
4.7 |
|
Mustard Seed Meal |
7.1 |
1.7* |
34* |
20* |
|
Mustard Seed Meal + Crab Meal |
7* |
1.8* |
32* |
12* |
*Student's T-Test; different from grower standard at 5% level of significance.
Table 1B. Soil analysis from December 7, 2016
|
Sample |
(P) |
(K) |
(Ca) |
(SO4) |
(Mg) |
(Mn) |
Fe |
Na in meq/L |
Cl in meq/L |
|
Methyl bromide grower standard |
51 |
148 |
3100 |
278 |
178 |
8.9 |
18 |
1.9 |
3.2 |
|
Mustard Seed Meal |
54 |
190* |
2933 |
318 |
193 |
19.2* |
16 |
1.5 |
1.9 |
|
Mustard Seed Meal + Crab Meal |
60 |
185* |
3100 |
589 |
150 |
20.1* |
16 |
1.5 |
1.9 |
*Student's T-Test, different from grower standard at 5% level of significance.
One sees immediately that the pH has fallen, even significantly, in plots treated with mustard seed meal and mustard seed meal + crab meal. This is not surprising, since in the month's time since the initial sample on Nov 7, the ammonium has clearly nitrified (releasing 2 H+ ions per molecule, in turn acidifying the soil) creating a big pool of nitrates which have gone up significantly over the grower standard.
EC has gone up a bit due to the higher nitrates (NOT because of sodium or chloride), and interestingly levels of manganese (Mn) a mineral sensitive to acidification apparently, have soared in both MSM treated plots. Levels of available potassium (K) have gone up significantly also in MSM treated plots.
Quite interesting on the whole. By the way, a soil report like this makes for pretty good reading, and outside of the EC which is for the time being a little high in the MSM plots, all the other numbers are right where I like to see them.
Stay tuned on this one; we are following all of this trial through the season.
- Author: Mark Bolda
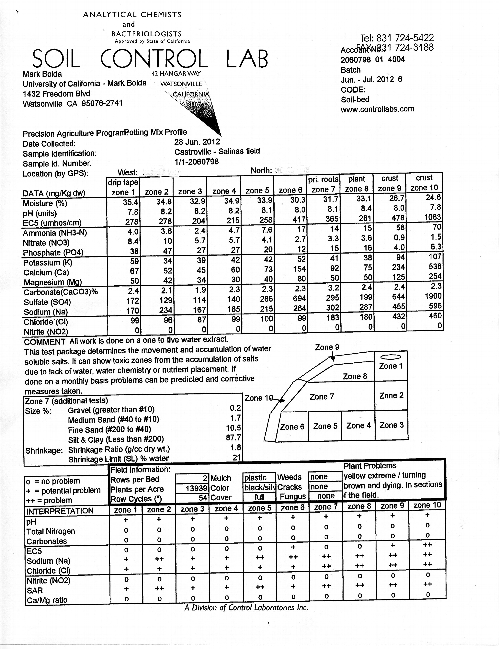
Introduction: The following is a description of a second, more thorough evaluation of the situation described previously in this space of yellow strawberry plants in a field in Castroville. This issue of yellow strawberry plants popping up in certain locations in the Castroville – Salinas production area has confounded us for years, but I believe the work described below allows us to make a strong argument as to the cause is of this malady at least in this case.
Materials and Methods: A total of four samples (two from an area of severe yellowing, and two from an area of apparently healthy green plants) were taken. Following the output example posted below (Figure 2), each sample consists of 10 zones of a bed, and each zone is tested for 14 parameters. At each soil sampling site, a representative plant sample was uprooted and taken away for analysis of tissue mineral concentration.
Results: The data in the Tables 1 and 2 below represent an average of the two samples taken each for yellow and green plants. In order to better interpret the data, several zones have been grouped together. Zones 9 and 10 represent the surface of the bed, zones 1, 2 and 3 represent the soil straight underneath the drip tape, zone 8 the plant zone, zone 7 the root zone and zones 6, 5 and 4 being underneath the root zone 7.
Table 1: Evaluation of Zones 1- 6 of Bed in Yellow and Healthy Areas
|
Data (mg/Kg dw) |
Zones 1,2 and 3 |
Zones 4,5 and 6 |
||
|
|
Yellow |
Healthy |
Yellow |
Healthy |
|
Moisture (%) |
33.7 |
33.6 |
29.8 |
31.8 |
|
pH (units) |
8.5 |
8.1 |
8.5 |
8.1 |
|
EC5 (umhos/cm) |
269 |
267 |
381 |
355 |
|
Ammonia (NH3-N) |
24 |
32 |
60 |
16 |
|
Nitrate (NO3) |
204 |
49 |
443 |
94 |
|
Phosphate (PO4) |
126 |
86 |
66 |
68 |
|
Potassium (K) |
157 |
184 |
157 |
92 |
|
Calcium (Ca) |
419 |
417 |
398 |
201 |
|
Magnesium (Mg) |
169 |
375 |
174 |
130 |
|
Carbonate (CaCO3) % |
2.3 |
1.8 |
2.7 |
1.7 |
|
Sulfate (SO4) |
219 |
195 |
694 |
412 |
|
Sodium (Na) |
454 |
417 |
578 |
426 |
|
Chloride (Cl) |
175 |
184 |
181 |
242 |
|
Nitrite (NO2) |
0 |
0 |
0 |
0 |
Table 2: Evaluation of Zones 7-10 of Bed in Yellow and Healthy Areas
|
Data (mg/Kg dw) |
Zone 7- Root Zone |
Zone 8- Plant Zone |
Zones 9 and 10 |
|||
|
|
Yellow |
Healthy |
Yellow |
Healthy |
Yellow |
Healthy |
|
Moisture (%) |
30.6 |
32.1 |
33 |
33.1 |
22.8 |
26.3 |
|
pH (units) |
8.7 |
8.2 |
8.7 |
8.3 |
8.3 |
8.2 |
|
EC5 (umhos/cm) |
305 |
389 |
258 |
374 |
938 |
546 |
|
Ammonia (NH3-N) |
34 |
14 |
54 |
16 |
48 |
44 |
|
Nitrate (NO3) |
314 |
30 |
199 |
25 |
968 |
452 |
|
Phosphate (PO4) |
6.5 |
57 |
85 |
55 |
111 |
45 |
|
Potassium (K) |
144 |
74 |
185 |
89 |
206 |
89 |
|
Calcium (Ca) |
341 |
156 |
713 |
194 |
512 |
195 |
|
Magnesium (Mg) |
140 |
83 |
383 |
127 |
384 |
104 |
|
Carbonate (CaCO3) % |
2 |
1.3 |
2.0 |
1.9 |
2.0 |
1.9 |
|
Sulfate (SO4) |
434 |
492 |
239 |
437 |
1216 |
622 |
|
Sodium (Na) |
500 |
446 |
492 |
496 |
872 |
520 |
|
Chloride (Cl) |
160 |
309 |
165 |
330 |
433 |
310 |
|
Nitrite (NO2) |
0 |
0 |
0 |
0 |
0 |
0 |
Table 3: Comparison of Mineral Concentrations of Leaf Tissue for Green and Yellow Plants
|
Mineral |
Yellow Plant |
Green Plant |
|
Total Nitrogen |
2.4% |
2.2% |
|
Total Phosphorous |
0.38% |
0.44% |
|
Potassium |
1.1% |
1.2% |
|
Calcium |
1.5% |
1.3% |
|
Magnesium |
0.55% |
0.38% |
|
Total Sulfur |
0.21% |
0.18% |
|
Copper |
4.5 ppm |
3.7 ppm |
|
Zinc |
23 ppm |
18 ppm |
|
Iron |
515 ppm |
365 ppm |
|
Manganese |
185 ppm |
108 ppm |
|
Boron |
73 ppm |
78 ppm |
|
Molybdenum |
1.1 ppm |
1.9 ppm |
|
Sodium |
350 ppm |
79 ppm |
|
Chloride |
4150 ppm |
3000 ppm |
I’ve also taken a look at the irrigation water. As is common in northern Monterey county, the farm gets its water as a mixture of recycled water blended with well or river water. A full report for an example of the blended irrigation water used on this farm is available from the Monterey Regional Water Pollution Control Agency at:
http://www.mrwpca.org/recycling/chem2012_blended.php
In the sample taken of the blended recycled and river water mix, conductivity (EC) was 1.3 dS/m, sodium 118 ppm, chloride 160 ppm and adjusted SAR of 3.4 (sodium adsorption ratio, an index of sodium hazard adjusted for the amount of calcium in the irrigation water).
Discussion: The pH of the soil in the beds in all zones is quite high, which is not surprising because of the high percentage of carbonates (lime) throughout. One can also see accumulations of nitrates, phosphates and potassium are substantially higher in the areas of the yellow plants, which quite likely due to their declining ability to take up the nutrients being continually applied as fertilizer. It is worth noting that nitrates in the high concentrations found in our soil tests can be toxic to plants, thus accelerating the decline of plants.
Nitrites, generated from ammonium in anaerobic conditions, are zero, indicating adequate aeration in the bed.
In terms of the irrigation water, referring to the water quality guidelines for crops developed by UC Cooperative Extension, we find that the water used on this farm can be used with some restriction to irrigate crops moderately susceptible to salinity such as strawberry. In other words, this irrigation water isn’t great but is OK.
It seems that the real culprits in this field are the accumulated amounts of chloride and sodium. Generally speaking, crops in our area perform best when the soil sodium levels are less than 250 ppm and soil chloride levels are less than 100 ppm.
Across all the soil samples the average amounts of sodium and chloride are above 250 ppm and 100 ppm respectively. High as they are, the concentrations of both ions do not vary greatly in the plant and root zones and the zones around them in either areas of green or yellow plants. There are, however, substantial differences in the concentrations of these ions at the crust (zones 9 and 10) between green and yellow plant areas. For example, on average soil from areas of yellow plants there is nearly a twofold accumulation of sodium in the crust of soil taken as well as a substantially higher amount of chloride over the soil sampled around green, healthy plants. This tells us that while these large amounts of sodium and chloride (also known as salts) are accumulating away from the plant via evaporation, they are nevertheless passing through more sensitive plant zones to get there. It is not difficult then to hypothesize that during this transition the yellow plants are picking up these salts obtaining the fourfold accumulation of sodium and 40% increase in chlorides that we observe in the leaf tissue of yellow plants over that of green.
Furthermore, it is notable that the amount of lime (CaCO3) in all the soil samples is high. This indicates that a lot of calcium coming into the soil from irrigation water is precipitating out and not as capable of sufficiently limiting the amount of exchangeable sodium. In turn the sodium hazard is high. This may also explain to some extent why areas quite close to one another respond differently, since in some areas (perhaps even quite close together) more calcium precipitates out than others.
My conclusion drawn from all this work in this particular instance of severe plant yellowing of strawberry plants is that the uptake of sodium and chloride by these affected plants is very high and they are being poisoned by salt.
Thank you to Frank Shields and Soil Control Lab for their generous assistance in this work. Thanks to other colleagues for their valuable insight and input which assisted me in the development of my conclusion.
This project was supported in part with funds provided by the California Strawberry Commission.

- Posted By: Mark Bolda
- Written by: Mark Bolda
As a postscript to last week’s post regarding salt and ammonium damage to area strawberry plantings, I will outline the results of the soil samples taken from a field demonstrating the symptoms described in that article.
Steve Koike and I collected soil samples from the affected field last Thursday, January 5. Soil samples were collected from four blocks, one of which had been overhead irrigated the day previous, and consisted of composites of at least five 5” deep samples taken from around the fertilizer band by the plant roots.
Samples were immediately taken to Soil Control Lab in Watsonville for analysis.
Results are as follows:
|
|
Nitrate (ppm) |
Ammonium (ppm) |
EC (dS/m) |
|
Sample 1 (not overhead irrigated): |
58 |
4.8 |
2.8 |
|
Sample 2 (not overhead irrigated): |
72 |
5.2 |
4.2 |
|
Sample 3 (not overhead irrigated): |
69 |
4.8 |
3.8 |
|
Sample 4 (overhead irrigated): |
24 |
5.1 |
2.2 |
The results are pretty clear in showing that the block (Sample 4 ) which had been watered by overhead irrigation had three times lower nitrate concentrations and about half the EC (which is electrical conductivity, a measure of salt) of the other three averaged as a group, but more equivocal on the reduction of ammonium.
To interpret the data in the table above, we can refer to work done some time ago which demonstrated EC’s in excess of 1.0 were related to loss in yield of strawberry, suggesting that real damage could occur at the 4x levels in the table above.

