- Author: Surendra K. Dara
- Author: Dave Peck, Manzanita Berry Farms

Botrytis cinerea infection appears as wilted flowers and a layer of spores on ripe fruit. Photo by Surendra Dara
Botrytis fruit rot or gray mold caused by Botrytis cinerea is an important disease of strawberry and other crops damaging flowers and fruits. Pathogen survives in the plant debris and soil and can be present in the plant tissues before flowers form. Infection is common on developing or ripe fruits as brown lesions. Lesions typically appear under the calyxes but can be seen on other areas of the fruit. As the disease progresses, a layer of gray spores forms on the infected surface. Severe infection in flowers results in the failure of fruit development. Cool and moist conditions favor botrytis fruit rot development. Sprinkler irrigation, rains, or certain agricultural practices can contribute to the dispersal of fungal spores.
Although removal of infected plant material and debris can reduce the source of inoculum in the field, regular fungicide applications are typically necessary for managing botrytis fruit rot. Since fruiting occurs continuously for several months and fungicides are regularly applied, botrytis resistance to fungicides is not uncommon. Applying fungicides only when necessary, avoiding continuous use of fungicides from the same mode of action group (check FRAC mode of action groups), exploring the potential of biological fungicides to reduce the risk of resistance development are some of the strategies for effective botrytis fruit rot management. In addition to several synthetic fungicides, several biological fungicides continue to be introduced into the market offering various options for the growers. Earlier field studies evaluated the potential of various biological fungicides and strategies for using them with synthetic fungicides against botrytis and other fruit rots in strawberry (Dara, 2019; Dara, 2020). This study was conducted to evaluate some new and soon to be released fungicides in fall-planted strawberry to support the growers, ag input industry, and to promote sustainable disease management through biological and synthetic pesticides.
Methodology
This study was conducted at the Manzanita Berry Farms, Santa Maria in strawberry variety 3024 planted in October, 2020. While Captan and Switch were used as synthetic standards, a variety of biological fungicides of microbial, botanical, and animal sources were included at various rates and different combinations and rotations. Products and active ingredients evaluated in this study included Captan Gold 4L (captan) from Adama, Switch 62.5 WG (cyprodinil 37.5% + fludioxinil 25%) from Syngenta, NSTKI-14 (potassium carbonate 58.04% + thyme oil 1.75%) from NovoSource, A22613 [A] (botanical extract) from Syngenta, Regalia (giant knotweed extract 5%) from Marrone Bio Innovations, EXP14 (protein 15-20%) from Biotalys, Gargoil (cinnamon oil 15% + garlic oil 20%) and Dart (caprylic acid 41.7% + capric acid 28.3%) from Westbridge, Howler (Pseudomonas chlororaphis strain AFS009), Theia (Bacillus subtilis strain AFS032321), and Esendo (P. chlororaphis strain AFS009 44.5% + azoxystrobin 5.75%) from AgBiome, ProBlad Verde (Banda de Lupinus albus doce – BLAD, a polypeptide from sweet lupine) from Sym-Agro with Kiplant VS-04 (chitosan 2.3%) or Nu-Film-P spreader/sticker, AS-EXP Thyme (thyme oil) from AgroSpheres, and AgriCell FunThyme (thyme oil) provided by AgroSpheres.
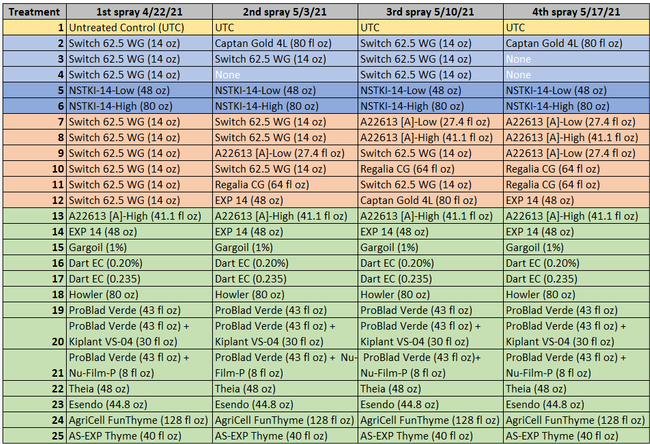
Table 1. List of treatments color coded according to the kind of fungicide (light blue=synthetic fungicide; dark blue=synthetic+biological fungicide active ingredient; peach=synthetic and biological fungicides alternated; green=biological fungicides)
Excluding the untreated control, rest of the 24 treatments can be divided into synthetic fungicides, a fungicide with synthetic + biological active ingredients (a formulation with two application rates), synthetic fungicides alternated with biological fungicides, and various kinds of biological fungicides (Table 1). Treatments were applied at a 7-10 day interval between 22 April and 17 May, 2021. Berries for pre-treatment disease evaluation were harvested on 19 April, 2021. Each treatment had a 5.67'X15' plot replicated four times in a randomized complete block design. Strawberries were harvested 3 days before the first treatment and 3-4 days after each treatment for disease evaluation. On each sampling date, marketable-quality berries were harvested from random plants within each plot during a 30-sec period and incubated in paper bags at outdoor temperatures under shade. Number of berries with botrytis infection were counted on 3 and 5 days after harvest (DAH) and percent infection was calculated. This is a different protocol than previous years' studies where disease rating was made on a 0 to 4 scale. Treatments were applied with a backpack sprayer equipped with Teejet Conejet TXVK-6 nozzle using 90 gpa spray volume at 45 PSI. Water was sprayed in the untreated control plots. Dyne-Amic surfactant at 0.125% was used for treatments that contained Howler, Theia, Esendo, AgriCell FunThyme, AS-EXP Thyme, and EXP 14. Research authorization was obtained for some products and crop destruction was implemented for products that did not have California registration.
Percent infection data were arcsine-transformed before subjecting to the analysis of variance using Statistix software. Significant means were separated using the least significant difference test.
Results
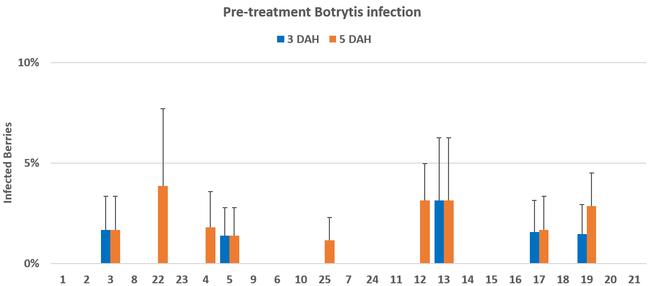
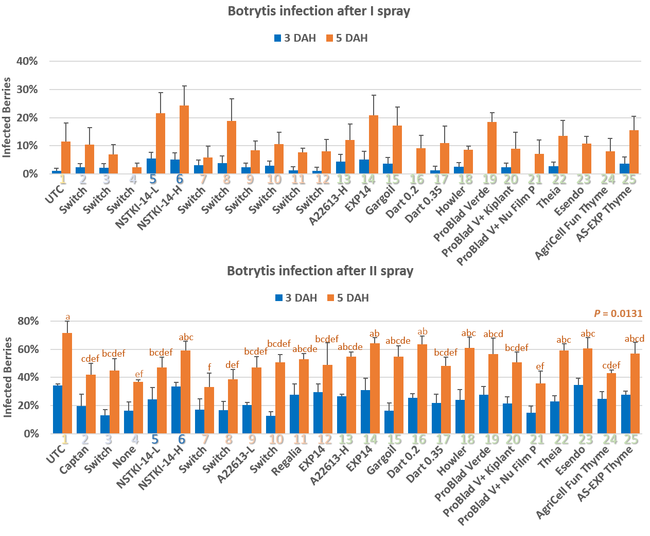
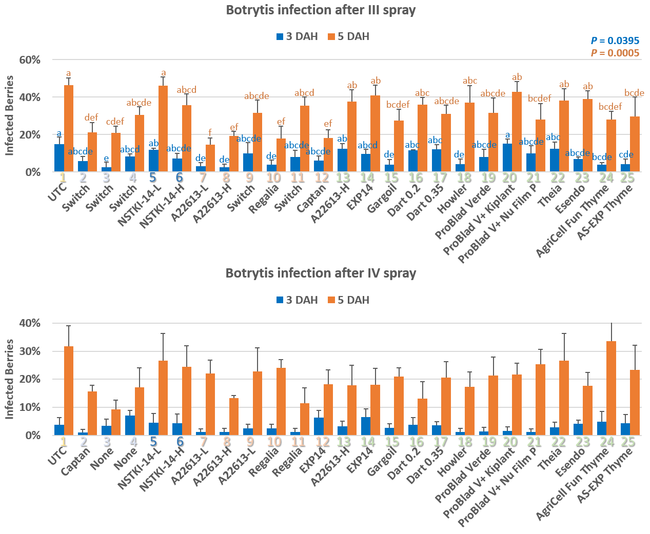
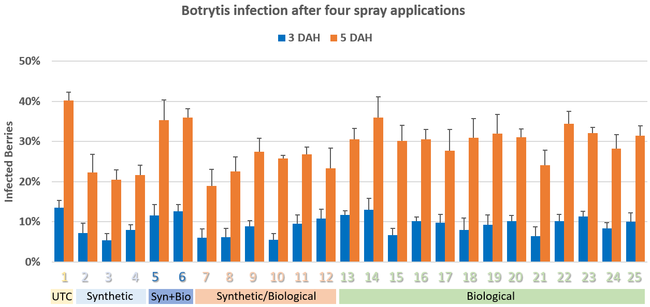
Pre-treatment infection was very low and occurred only in some treatments with no statistical difference (P > 0.05). Infection levels increased for the rest of the study period. There was no statistically significant difference (P > 0.05) among treatments for disease levels 3 or 5 days after the first spray application. Differences were significant (P = 0.0131) in disease 5 DAH after the second spray application where 13 treatments from all categories had significantly lower infection than the untreated control. After the third spray application, infection levels were significantly lower in eight treatments in 3 DAH observations (P = 0.0395) and 10 treatments in 5 DAH observations (P = 0.0005) compared to untreated control. There were no statistical differences (P > 0.05) among treatments for observations after fourth spray application or for the average of four applications. However, there were numerical differences where infection levels were lower in several treatments than the untreated control plots.
In general, the efficacy of both synthetic and biological fungicides varied throughout the study period among the treatments. When the average for post-treatment observations was considered, infection was numerically lower in all treatments regardless of the fungicide category. Multiple biological fungicide treatments either alone or in rotation with synthetic fungicides appeared to be as effective as synthetic fungicides.
Conclusions
Botanical and microbial fungicides can be effective against either for using alone or in rotation with synthetic fungicides for suppressing botrytis fruit rot in strawberry. Additional studies can help optimize the application rates and use strategies for those fungicides that were not as effective as others. Sanitation practices and use of synthetic and biological fungicides help manage botrytis fruit rot.
Acknowledgements: Thanks to AgBiome, AgroSpheres, Biotalys, NovaSource, Sym-Agro, Syngenta, and Westbridge for funding and Chris Martinez for his technical assistance.
References
Dara, S. K. 2019. Five shades of gray mold control in strawberry: evaluating chemical, organic oil, botanical, bacterial, and fungal active ingredients. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=30729
Dara, S. K. 2020. Evaluating biological fungicides against botrytis and other fruit rots in strawberry. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=43633
- Author: Surendra K. Dara
Strawberry, a high-value specialty crop in California, suffers from several soilborne, fruit, and foliar diseases. Verticillium wilt caused by Verticillium dahliae, Fusarium wilt caused by Fusarium oxysporum f. sp. fragariae, and Macrophomina crown rot or charcoal rot caused by Macrophomina phaseolina are major soilborne diseases that cause significant losses without proper control. Chemical fumigation, crop rotation with broccoli, nutrient and irrigation management to minimize plant stress, and non-chemical soil disinfestation are usual control strategies for these diseases. Botrytis fruit rot or gray mold caused by Botrytis cineaea is a common fruit disease requiring frequent fungicidal applications. Propagules of gray mold fungus survive in the soil and infect flowers and fruits. A study was conducted to evaluate the impact of drip application of various fungicides on improving strawberry health and enhancing fruit yields.
Methodology
This study was conducted in an experimental strawberry field at the Shafter Research Station during 2019-2020. Cultivar San Andreas was planted on 28 October 2019. No pre-plant fertilizer application was made in this non-fumigated field which had Fusarium wilt, Macrophomina crown rot, and Botrytis fruit rot in the previous year's strawberry planting. Each treatment was applied to a 300' long bed with single drip tape in the center and two rows of strawberry plants. Sprinkler irrigation was provided immediately after planting along with drip irrigation, which was provided one or more times weekly as needed for the rest of the experimental period. Each bed was divided into six 30' long plots, representing replications, with an 18' buffer in between. Between 6 November 2019 and 9 May 2020, 1.88 qt of 20-10-0 (a combination of 32-0-0 urea ammonium nitrate and 10-34-0 ammonium phosphate) and 1.32 qt of potassium thiosulfate was applied 20 times at weekly intervals through fertigation. Treatments were applied either as a transplant dip or through the drip system using a Dosatron fertilizer injector (model D14MZ2). The following treatments were evaluated in this study:
i) Untreated control: Neither transplants nor the planted crop was treated with any fungicides.
ii) Abound transplant dip: Transplants were dipped in 7 fl oz of Abound (azoxystrobin) fungicide in 100 gal of water for 4 min immediately prior to planting. Transplant dip in a fungicide is practiced by several growers to protect the crop from fungal diseases.
iii) Rhyme: Applied Rhyme (flutriafol) at 7 fl oz/ac immediately after and 30, 60, and 90 days after planting through the drip system.
iv) Velum Prime with Switch: Applied Velum Prime (fluopyram) at 6.5 fl oz/ac 14 and 28 days after planting followed by Switch 62.5 WG (cyprodinil + fludioxinil) at 14 oz/ac 42 days after planting through the drip system.
v) Rhyme with Switch: Four applications of Rhyme at 7 fl oz/ac were made 14, 28, 56, and 70 days after planting with a single application of Switch 62.5 WG 42 days after planting through the drip system.
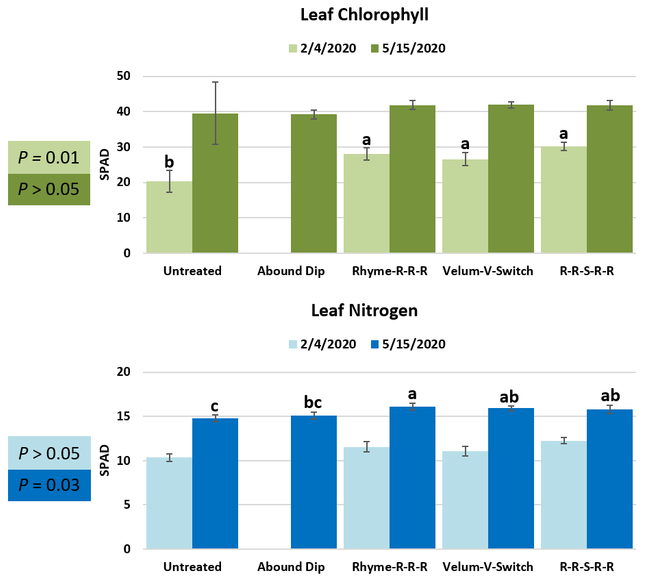
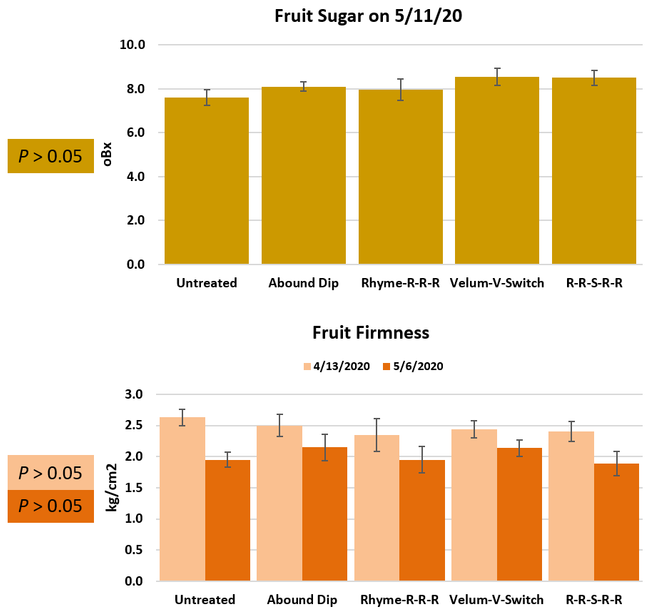
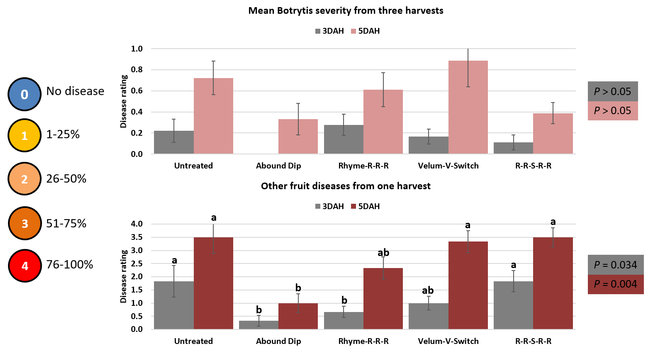
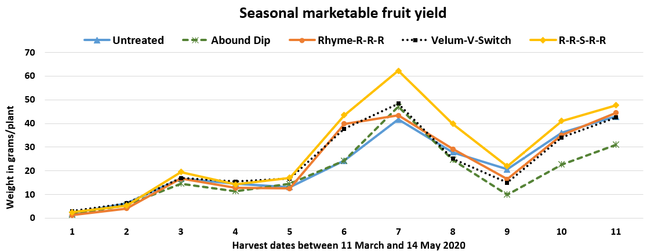
Parameters observed during the study included leaf chlorophyll and leaf nitrogen (with chlorophyll meter) in February and May; fruit sugar (with refractometer) in May; fruit firmness (with penetrometer) in April and May; severity of gray mold (caused by Botrytis cinereae) twice in March and once in May, and other fruit diseases (mucor fruit rot caused by Mucor spp. and Rhizopus fruit rot caused by Rhizopus spp.) once in May 3 and 5 days after harvest (on a scale of 0 to 4 where 0=no infection; 1=1-25%, 2=26-50%, 3=51-75% and 4=76-100% fungal growth); and fruit yield per plant from 11 weekly harvests between 11 March and 14 May 2020. Leaf chlorophyll and nitrogen data for the Abound dip treatment were not collected in February. Data were analyzed using analysis of variance in Statistix software and significant means were separated using the Least Significant Difference test.
Results and Discussion
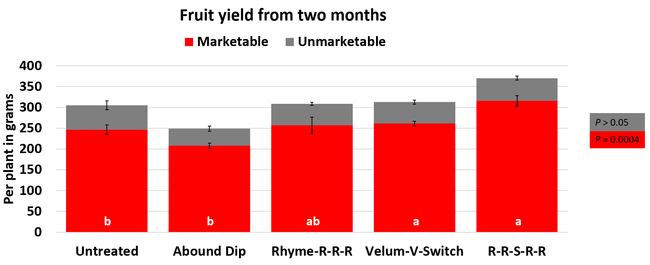
Leaf chlorophyll content was significantly higher in plants that received drip application of fungicides compared to untreated plants in February while leaf nitrogen content was significantly higher in the same treatments during the May observation. There were no differences in fruit sugar or average fruit firmness among the treatments.
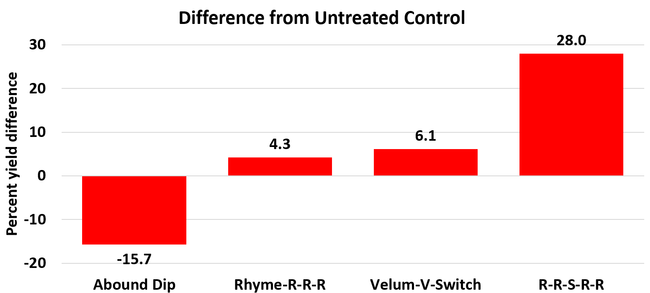
The average gray mold severity from three harvest dates was low and did not statistically differ among the treatments. However, the severity of other diseases was significantly different among various treatments with the lowest rating in Abound transplant dip on both 3 and 5 days after harvest and only 3 days after harvest in plants that received four applications of Rhyme. Unlike the previous year, visible symptoms of the soilborne diseases were not seen during the study period to evaluate the impact of the treatments. However, there were significant differences among treatments for the marketable fruit yield. The highest marketable yield was observed in the treatment that received Rhyme and Switch followed by Velum Prime and Switch and Rhyme alone. The lowest fruit yield was observed in Abound dip treatment. Unmarketable fruit (deformed or diseased) yield was similar among the treatments. Compared to the untreated control, Abound dip resulted in 16% less marketable yield and such a negative impact from transplant dip in fungicides has been seen in other studies (Dara and Peck, 2017 and 2018; Dara, 2020). Marketable fruit yield was 4-28% higher where fungicides were applied to the soil.
Although visible symptoms of soilborne diseases were absent during the study, periodic drip application of the fungicides probably suppressed the fungal inocula and associated stress and might have contributed to increased yields. The direct impact of fungicide treatments on soilborne pathogens was, however, not clear in this study due to the lack of disease symptoms. Considering the cost of chemical fumigation or soil disinfestation and the environmental impact of chemical fumigation, treating the soil with fungicides can be an economical option if they are effective. While this study presents some preliminary data, additional studies in non-fumigated fields in the presence of pathogens are necessary to consider soil fungicide treatment as a control option.
Acknowledgments: Thanks to FMC for funding this study and Marjan Heidarian Dehkordi and Tamas Zold for their technical assistance.
References
Dara, S. K. 2020. Improving strawberry yields with biostimulants and nutrient supplements: a 2019-2020 study. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=43631
Dara, S. K. and D. Peck. 2017. Evaluating beneficial microbe-based products for their impact on strawberry plant growth, health, and fruit yield. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=25122
Dara, S. K. and D. Peck. 2018. Evaluation of additive, soil amendment, and biostimulant products in Santa Maria strawberry. CAPCA Adviser, 21 (5): 44-50.
- Author: Surendra K. Dara
Balanced nutrient inputs are essential for optimal plant growth and yields. Depending on the soil, crop, and environmental conditions, certain nutritional supplements further enhance crop performance. While macro- and micro-nutrients are necessary for plant growth and optimal yields, biostimulants play multiple roles by increasing the bioavailability of nutrients, improving nutrient and water absorption, protecting plants from pestiferous organisms either through direct antagonism or by triggering plants defense mechanisms (Berg, 2009; Dara, 2019a). In addition to improving health and yields, biostimulants are also known to increase nutritional quality (Parađiković et al., 2011; Fierentino et al., 2018). Multiple field studies in California demonstrated the potential of biostimulants and soil amendments in improving yields in tomato (Dara, 2019b; Dara and Lewis, 2019) and strawberry (Dara and Peck, 2018; Dara, 2019a). As the knowledge of biostimulants and their potential for sustainable agriculture is expanding, there has been a steady introduction of biostimulant products in the market warranting additional studies. A study was conducted to evaluate the potential of different biostimulant materials on strawberry growth, health, and fruit yields.
Methodology
This study was conducted in an experimental strawberry field at the Shafter Research Station during 2019-2020. Cultivar San Andreas was planted on 29 October 2019. No pre-plant fertilizer application was made in this non-fumigated field which had both Fusarium oxysporum and Macrophomina phaseolina infections in previous year's strawberry planting. Each treatment was applied to a 300' long bed with single drip tape in the center and two rows of strawberry plants. Sprinkler irrigation was provided immediately after planting along with drip irrigation, which was provided one or more times weekly as needed for the rest of the experimental period. Each bed was divided into six 30' long plots, representing replications, with an 18' buffer in between. This study included both biostimulant and nutrient supplements, but this article presents data from the biostimulant treatments only. Treatments were applied either as fertigation through the drip system using a Dosatron or sprayed over the plants with a handheld garden sprayer. The following treatments were evaluated in this study:
i) Grower Standard (GS): Between 6 November 2019 and 9 May 2020, 1.88 qt of 20-10-0 (a combination of 32-0-0 urea ammonium nitrate and 10-34-0 ammonium phosphate) and 1.32 qt of potassium thiosulfate was applied 20 times at weekly intervals through fertigation. This fertilizer program was used as the standard for all treatments except for the addition of biostimulant materials.
ii) GS + Abound: Transplants were dipped in 7 fl oz of Abound (azoxystrobin) fungicide in 100 gal of water for 4 min immediately prior to planting. Transplant dip in a fungicide is practiced by several growers to protect from fungal diseases and is considered as another standard in this study.
iii) GS + Locus program: Applied Str10 (Wickerhamomyces sp.) at 5 fl oz/ac with molasses at 10 fl oz/ac immediately after planting and Rhizolizer (Trichoderma harzianum and Bacillus amyloliquefaciens) at 3 fl oz with a food source blend at 10 fl oz 2 weeks after Str10 application through the drip system. Repeated the same pattern starting from mid-February 2020. From February to May, applied 6 fl oz/ac of Rhizolizer with 20 fl oz/ac of food source once a month. Str10 is an unregistered product with yeast that is expected to help with nutrient uptake and phosphorous mobilization for improved plant vigor and yield. Rhizolizer is expected to solubilize soil nutrients and improve crop growth and yield.
iv) GS + Redox program: Starting from about one month after planting, diKaP (0-31-50 NPK) was applied as a foliar spray at 2 lb in 50 gpa every two weeks. In addition to potassium and phosphorus, diKaP also contains proprietary soluable carbon compounds that improve antioxidant production leading to increased plant respiration and tolerance to abiotic stress.
v) Bio Huma Netics (BHN) program: Transplants were dipped in 10 gal of water with 6.4 fl oz of BreakOut (4-14-2 NPK), 1.28 fl oz of Promax (thyme oil), 1.28 fl oz of Vitol (8-16-4 NPK with iron, manganese, sulfur, and zinc), and 1.28 fl oz of Zap (8-0-0 N with iron, manganese, sulfur and zinc) for 4 min immediately prior to planting. Custom blends of macro- and micro-nutrients (Ultra Precision A and B) were prepared based on soil (pre-planting) and plant tissue analyses and applied as a substitute to the grower standard fertility program. Ultra Precision A during the first 30 days after planting and Ultra Precision B for the rest of the study period were applied at weekly intervals at 1.6 gal/bed for a total of 12 times (compared to 20 fertigation events for the grower standard program). Ultra Precision blends were made with Super Phos/Phos-Max, Super Potassium, X-Tend, Nitric acid, Calcium, 44 Mag, BreakOut, Vitol, Max Pak, Iro-Max, Activol, Comol, and Surf-Max that provided N, P, and K along with boron, calcium, cobalt, copper, iron, magnesium, manganese, molybdenum, and sulfur.
vi) GS + BioWorks program 1: Applied 32 fl oz of ON-Gard (based on soy protein hydrolysate) every two weeks through the drip system from planting until canopy develops and then applied as a foliar spray in 50 gpa. ON-Gard is expected to increase the nutrient use efficiency and decrease abiotic stress to the plants.
vii) GS + BioWorks program 2: Applied 32 fl oz of ON-Gard (soy protein-based) every two weeks through the drip system from planting until canopy develops and then sprayed in 50 gpa. Also applied RootShield Plus WP (T. harzianum and T. virens) at 2 lb/ac through drip immediately after planting and 1 lb/ac at the end of November and again at the end of December 2019. RootShield is a biofungicide expected to protect strawberry from phytopathogens and improve water and nutrient uptake.
viii) GS + Fauna Soil Production (FSP) program: Applied CropSignal at 10 gpa six days prior to planting and at 5 gpa 30 after transplanting through the drip system. CropSignal is a carbon-based nutrient formula containing botanical extracts and along with cobalt, copper, manganese, and zinc and is expected to support the growth and diversity of beneficial aerobic soil microbes for improved soil structure, water retention, nutrient cycling, and plant protection.
ix) GS + Stoller program 1: Applied Stoller Root Feed Dry (9-0-5 NPK with boron, calcium, magnesium, and molybdenum) at 10 lb/ac every 10 days starting from 19 February 2020 and Stoller Grow (4-0-3 NPK with copper, magnesium, manganese, and zinc) at 8 fl oz/ac once on 27 February 2020 through the drip system. Stoller Root Feed Dry is expected to promote continuous root growth by maintaining nutritional balance while Stoller Grow is expected to increase growth efficiency and abiotic stress tolerance.
x) GS + Stoller program 2: Applied Harvest More Urea Mate (5-10-27 NPK with boron, calcium, cobalt, copper, magnesium, manganese, molybdenum, and zinc) at 10 lb/ac along with Stoller Crop Mix (algal extract with boron and calcium) at 8 fl oz/ac every 10 days starting from 19 February 2020 and Stoller Grow at 8 fl oz/ac once on 27 February 2020 through the drip system. Harvest More Urea Mate is expected to provide optimal plant growth while Stoller Crop Mix is expected to maintain the nutritional balance and improve crop vigor and yields.
Parameters observed during the study included canopy growth (area of the canopy) in January, February, and March; first flower and fruit count in January; leaf chlorophyll and leaf nitrogen (with chlorophyll meter) in January, February, and May; fruit sugar (with refractometer) in March and May; fruit firmness (with penetrometer) in March, April, and May; severity of gray mold (caused by Botrytis cinereae) and other fruit diseases (mucor fruit rot caused by Mucor spp. and Rhizopus fruit rot caused by Rhizopus spp.) 3 and 5 days after harvest (on a scale of 0 to 4 where 0=no infection; 1=1-25%, 2=26-50%, 3=51-75% and 4=76-100% fungal growth) in March and May; sensitivity to heat stress (expressed as the number of dead and dying plants) in May; and fruit yield per plant from 11 weekly harvests between 11 March and 14 May 2020. Data were analyzed using analysis of variance in Statistix software and significant means were separated using the Least Significant Difference test.
Results and Discussion
The impact of treatments varied on various measured parameters. The interactions among plants, available nutrients, beneficial and pathogenic microorganisms in the crop environment, the influence of environmental factors, and how all these biotic and abiotic factors ultimately impact the crop health and yields are very complex. The scope of this study was only to measure the impact of biostimulants and nutrient supplements on growth, health, and yield parameters and not to investigate those complex interactions.
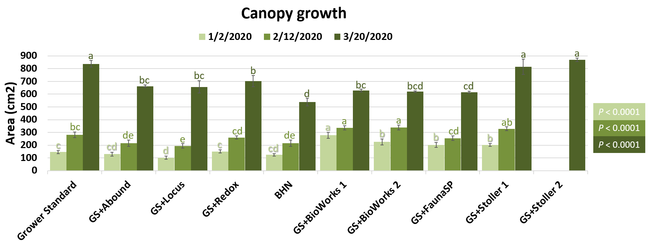
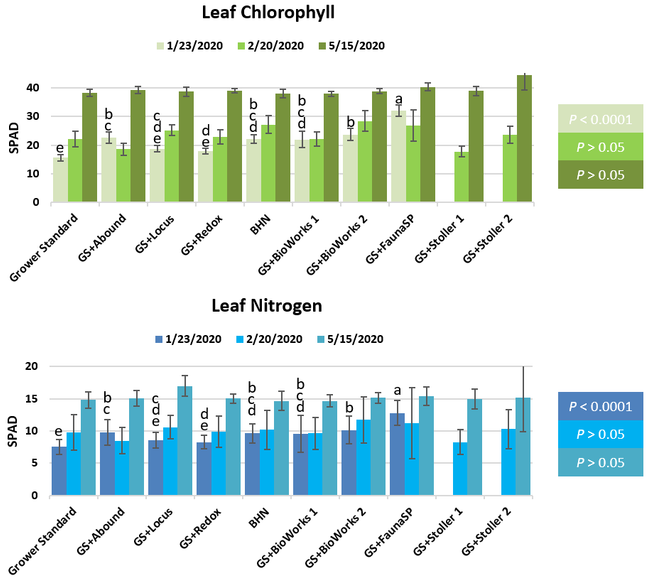
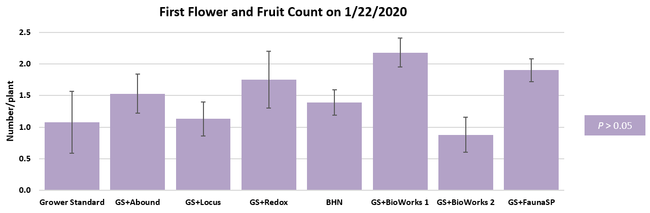
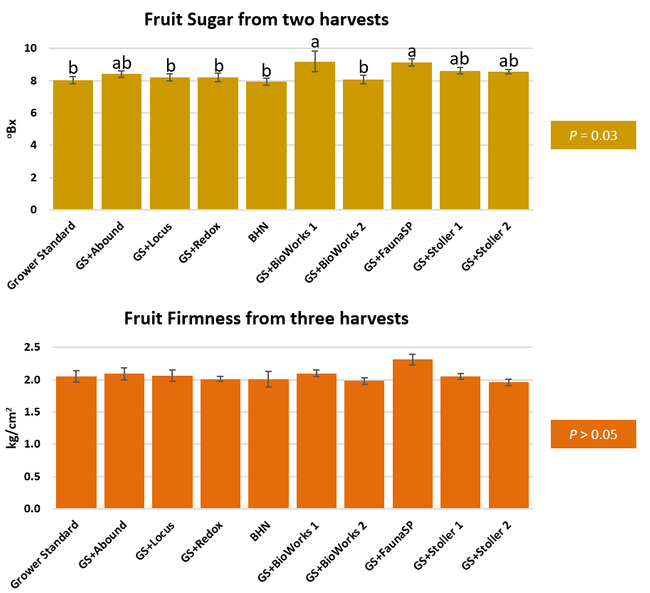
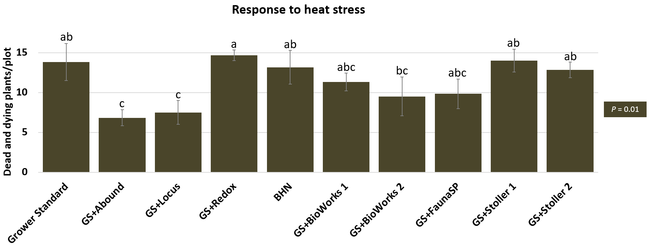
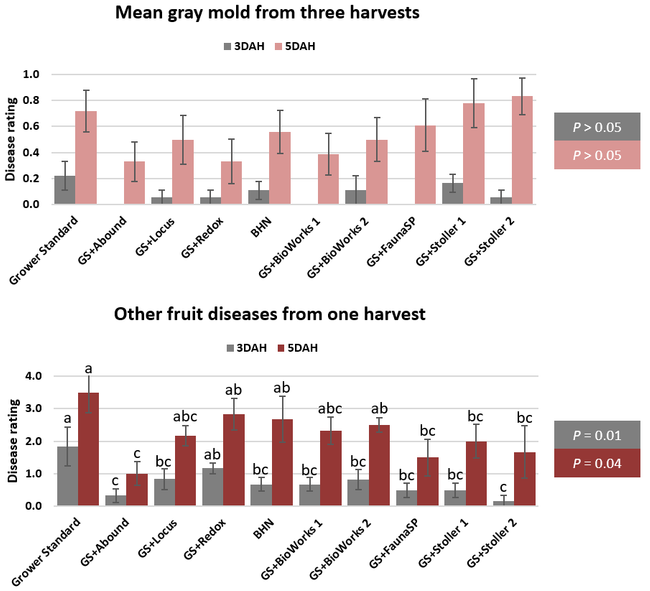
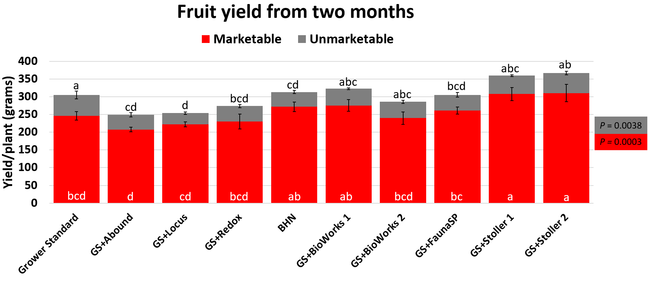
The canopy size does not always correspond with yields but could be indicative of stresses and how the plant is responding to them in the presence of treatment materials. Plants in some treatments had significantly larger canopy size in January and February, but plants in the grower standard and both Stoller programs were significantly larger than the rest by March. Leaf chlorophyll and nitrogen contents were significantly different among treatments only in January where the grower standard plants had the lowest and the plants that received CropSignal had the highest. When the counts of the first onset of flowers and developing fruits were taken in January, plants that received the BioWorks program that only received ON-Gard had the highest number followed by the CropSignal and Abound treatments. Stoller treatments were not included in the study at this time, so data for leaf chlorophyll, nitrogen, and first flower and fruit counts were not available in January. Average fruit sugar was the highest in BioWorks program with ON-Gard alone followed by FSP's Crop Signal, both Stoller programs, and the Abound treatments. There was no statistically significant difference in the average fruit firmness among the treatments. Severity of the gray mold, which occurred at low levels during the observation period, also did not statistically differ among the treatments. However, the severity of other diseases was significantly different among various treatments with the highest level in fruits from the grower standard. Temperatures were unusually high during the last week of May and several plants exhibited heat stress and started to die. The number of dead or dying plants on 28 May was the lowest in Locus and Abound treatments.
There were significant differences in marketable and unmarketable fruit yields among treatments. Highest marketable yields were seen in both Stoller treatments followed by BioWorks program with ON-Gard alone, BHN, and other treatments. Transplant dip in a fungicide seems to have a negative impact on fruit yields as observed in the current study or earlier studies (Dara and Peck, 2017 and 2018; Peck unpublished data). While the grower standard had the highest amount of unmarketable fruits, the Locus treatment had the lowest in this study. Fruit yield and some of the observed parameters appeared to be better in the grower standard compared to some treatments, which has also been seen in some earlier strawberry studies. While biostimulants can help plants under some stresses, providing sufficient macro- and micro-nutrients seems to be critical for higher fruit yields as seen with Stoller and BHN treatments. It is important to note that BHN materials were applied only 12 times compared to 20 applications of the grower standard treatment or other treatments that were applied on top of the grower standard treatment. It is also important to note that when ON-Gard was used alone, it also improved the marketable fruit yields by nearly 12% compared to the grower standard. When marketable fruit yield in the Abound treatment was considered, all treatments performed better 7-50% higher yields. Sometimes natural balance of the nutrients, organic matter, and microbial community in the soil might result in optimal yields in the absence of pathogens or other stressors. However, it is very common to use fungicidal treatments or add biological or supplemental nutrition to protect from potential threats and improving yields. These results help understand the impact of various biostimulants and supplements and warrant the need to continue such studies under various environmental, crop, and soil conditions.
Acknowledgments: Thanks to Bio Huma Netics, BioWorks, Inc., Fauna Soil Production, Locus Agricultural Solutions, Redox Ag, and Stoller for the financial support of the study and Marjan Heidarian Dehkordi and Tamas Zold for their technical assistance.
References
Berg, G. 2009. Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 84: 11-18.
Dara, S. K. 2019a. Improving strawberry yields with biostimulants: a 2018-2019 study. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=31096
Dara, S. K. 2019b. Effect of microbial and botanical biostimulants with nutrients on tomato yield. CAPCA Adviser, 22(5): 40-45.
Dara, S. K. and D. Peck. 2017. Evaluating beneficial microbe-based products for their impact on strawberry plant growth, health, and fruit yield. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=25122
Dara, S. K. and D. Peck. 2018. Evaluation of additive, soil amendment, and biostimulant products in Santa Maria strawberry. CAPCA Adviser, 21 (5): 44-50.
Dara, S. K. and E. Lewis. 2019. Evaluating biostimulant and nutrient inputs to improve tomato yields and crop health. Progressive Crop Consultant 4(5): 38-42.
Fiorentino, N., V. Ventorino, S. L. Woo, O. Pepe, A. De Rosa, L. Gioia, I. Romano, N. Lombardi, M. Napolitano, G. Colla, and Y. Rouphael. 2018. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Frontiers in Plant Sci. 9: 743.
Parađiković, N., T. Vinković, I. V. Vrček, I. Žuntar, M. Bojić, and M. Medić-Šarić. 2011. Effect of natural biostimulants on yield and nutritional quality: an example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food. Agric. 91: 2146-2152.
- Author: Surendra K. Dara
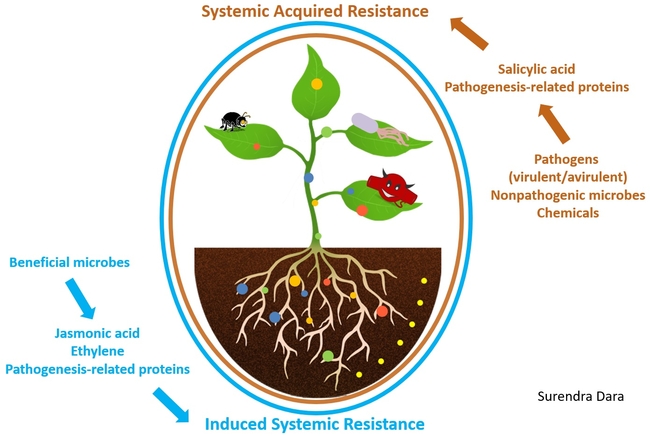
Biostimulants are beneficial microorganisms or substances that can be used in crop production to improve plants' immune responses and their ability to perform well under biotic and abiotic stresses. Biostimulants induce plant resistance to stress factors through systemic acquired resistance or induced systemic resistance. When plants are exposed to virulent and avirulent pathogens, non-pathogenic microorganisms, and some chemicals, the systemic acquired resistance mechanism is activated through the salicylic acid pathway triggering the production of pathogenesis-related proteins. On the other hand, when plants are exposed to beneficial microbes, the induced systemic resistance mechanism is activated through the jasmonic acid and ethylene pathways. The jasmonic acid pathway also leads to pathogenesis-related protein production in plants. In other words, when plants are exposed to pathogens, non-pathogens, or other compounds, various defense genes are activated through two major immune responses, helping plants fight the real infection or prepare them for potential infection. Beneficial microbes and non-microbial biostimulants are like vaccines that prepare plants for potential health problems.
Earlier studies in tomato (Dara and Lewis, 2018; Dara, 2019a) and strawberry (Dara and Peck, 2018; Dara, 2019b) demonstrated varying levels of benefits to crop health and yield improvements from a variety of botanical, microbial, or mineral biostimulants and other supplements. Some of the evaluated products resulted in significant yield improvement in both tomatoes and strawberries compared to the grower standard practices. There are several biostimulant products in the market with a variety of active ingredients, and some also have major plant nutrients such as nitrogen, phosphorus, and potassium. Depending on the crop, growing conditions, potential risk of pests and diseases, and other factors, growers can use one or more of these products. A study was conducted to evaluate the impact of various biostimulants on the yield, quality, and shelf life of strawberries.
Methodology
Strawberry cultivar San Andreas was planted late November 2018 and treatments were administered at the time of planting or soon after, depending on the protocol. Each treatment had a 290' long strawberry bed where 10' of the bed at each end was left out as a buffer. Then, six 30' long plots, each representing a replication, were marked within each bed with an 18' buffer between the plots. Since the test products needed to be applied through the drip system, an entire bed was allocated for each treatment, except for the standard program that had one bed on either side of the experimental block, and plots were marked within each bed for data collection. The following treatment regimens were used in the study:
1. Standard Program (SP): Major nutrients were provided in the form of Urea Ammonium Nitrate Solution 32-0-0, Ammonium Polyphosphate Solution, and Potassium Thiosulfate (KTS 0-0-25). Nitrogen, phosphorus, and potassium were applied before planting in November 2018 at 170, 60, and 130 lb/acre, respectively. From 15 January to 9 May 2019, a total of 26 lb of nitrogen, 13 lb of phosphorus, and 26 lb of potassium were applied through 13 periodic applications.
2. SP + Terramera Program: Formulation labeled as Experimental A (cold-pressed neem 70%) was applied at 1.2% vol/vol immediately after planting. Additional applications were made starting from 2 weeks after planting once every two weeks until the end of February (six times), followed by 13 weekly applications from the beginning of March.
3. SP + Locus Low Rate Program: This program contained Rhizolizer soil amendment (Trichoderma harzianum 1X108 CFU/ml and Bacillus amyloliquefaciens 1X109 CFU/ml) at 3 fl oz/acre, humic acid at 13.5 fl oz/acre, and kelp at 6.8 fl oz/acre. The first application was made within 15 days and at 30 days after planting followed by once in February, March, and April 2019.
4. SP + Locus High Rate Program: This program contained Rhizolizer soil amendment (Trichoderma harzianum 1X108 CFU/ml and Bacillus amyloliquefaciens 1X109 CFU/ml) at 6 fl oz/acre, humic acid at 13.5 fl oz/acre, and kelp at 6.8 fl oz/acre. The first application was made within 15 days and at 30 days after planting followed by once in February, March, and April 2019.
5. SP + BioGro Program: Transplants were treated with Premium Plant BB (Beauveria bassiana 1.1%) by spraying 2 fl oz/acre (1.29 ml in 850 ml of water). About 7 weeks after planting, 30 gpa of Plant-X Rhizo-Pro (botanical extracts), 2 gpa of CHB Premium 21 (humic acid blend), 3 gpa of CHB Premium 6 (3% humic acids), and 5 gpa of NUE Flourish 4-12-0 were applied. Starting from mid-February 2019, 15 gpa of Plant-X Rhizo-Pro, 1 gpa of CHB Premium 21, and 2 gpa of CHB Premium 6 were applied four times every 2 weeks until the end of March. Starting from 5 April 2019, 8 weekly applications of 10 gpa of Plant-X Rhizo-Pro, 1 gpa of CHB Premium 21, 2 gpa of Premium 6, and 4 gpa of NUE Flourish 4-12-0 were made until 26 May 2019.
6. SP + Actagro Program: Structure 7-21-0 at 3 gpa and Liquid Humus 0-0-4 with 22% organic acids at 1 gpa were first applied within 1 week of planting and then three more times every 2 weeks until the end of December 2018. Additional monthly applications were made from the end of January to the end of April 2019.
All the fertilizers and treatment materials were applied through the drip system using the Dosatron (Model D14MZ2) equipment. The following parameters were measured during the experimental period from January to May 2019.
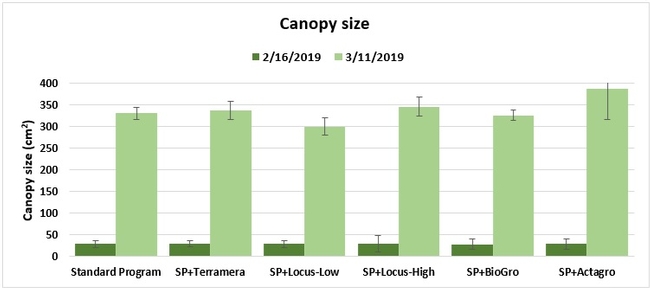
Canopy: The size of the plant canopy was determined on 21 January and again on 17 February 2019 by measuring the spread of the canopy across and along the length of the bed from 16 random plants within each plot, and calculating the area.
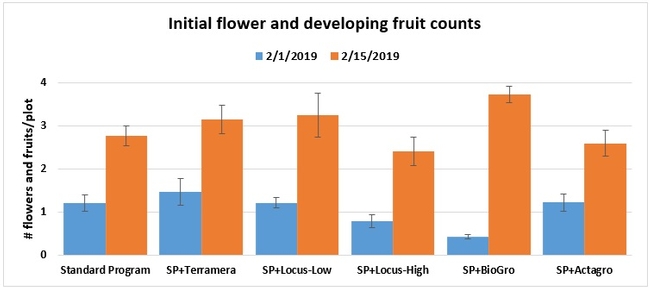
Initial flowering and fruiting: When flowering initiated, the number of flowers and developing fruits was counted from 16 random plants within each plot on 1 and 16 February 2019.
Fruit yield: Fruit was harvested weekly from every plant within each plot from 3 March to 26 May 2019 on 11 dates and the number and weight of the marketable and unmarketable fruit was determined. Due to a technical error, some of the yield data from an additional date (29 March) were lost and excluded from the analysis.
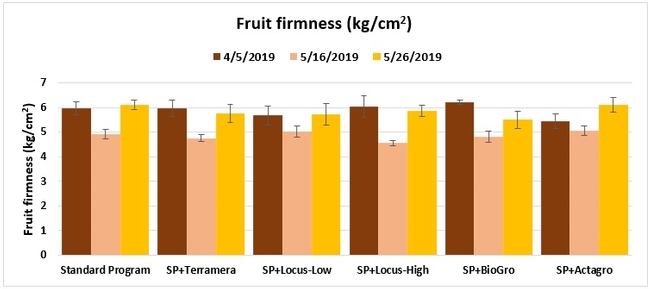
Fruit firmness: The firmness of two marketable fruit from each of five random plants per plot was measured using a penetrometer on 5 April, and 16 and 26 May 2019.
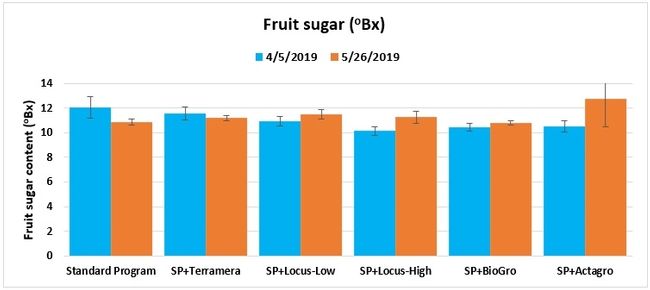
Fruit sugar content: The sugar content from one marketable fruit from each of 10 plants per plot was measured using a refractometer on 5 April and 26 May 2019.
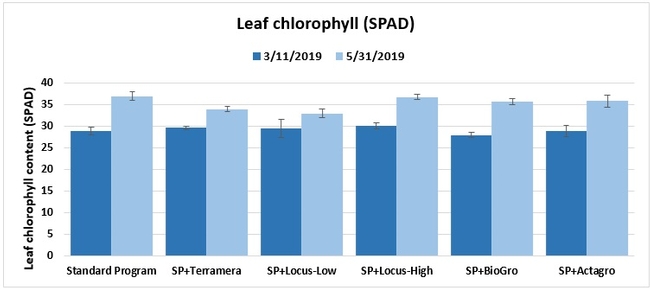
Leaf chlorophyll content: On 11 March and 31 May 2019, the chlorophyll content of one mature leaf from each of five random plants per plot was measured using a chlorophyll meter.
Postharvest disease: Marketable fruit harvested on 21 and 28 April, and 5 and 26 May 2019 was kept at the room temperature in perforated plastic containers (clamshells) and the growth of gray mold (Botrytis cinerea) or Rhizopus fruit rot fungus (Rhizopus spp.) was measured on a scale of 0 to 4 (where 0=no fungus, 1=1-25%, 2=26-50%, 3=51-75%, and 4=76-100% fungal growth) 3 and 5 days after each harvest.
Data were analyzed using analysis of variance in Statistix software and significant means were separated using the Least Significant Difference means separation test.
Results and Discussion
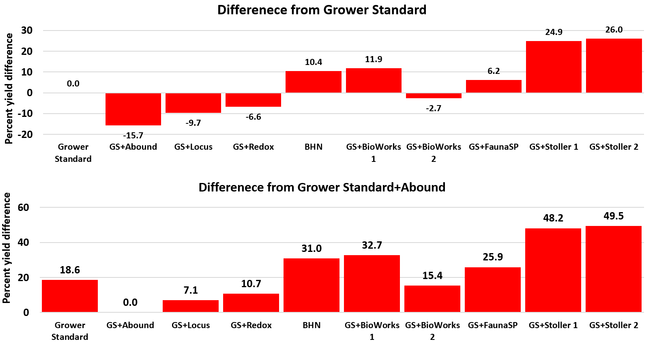
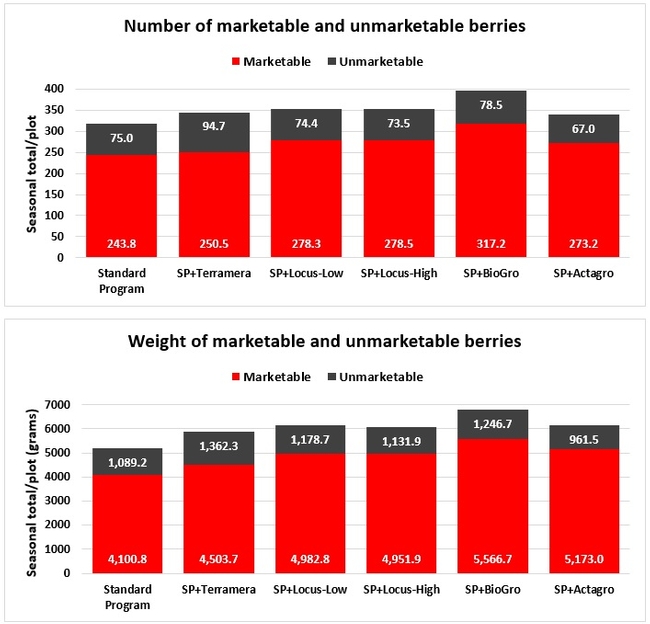
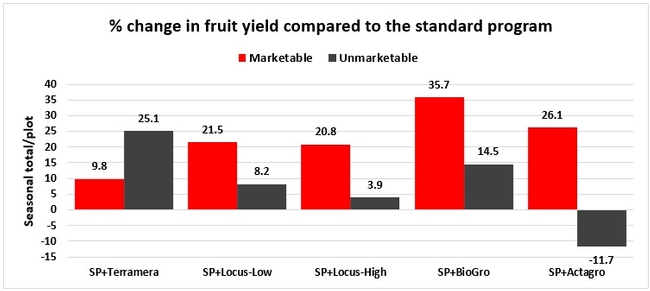
Statistically significant differences among treatments were seen for the seasonal total number of unmarketable berries (P = 0.0172), the initial flower and fruit numbers on 1 February (P < 0.0014), the leaf chlorophyll content on 31 May (P = 0.0144), and the disease rating 3 days after the 28 April harvest (P = 0.0065).
Treatments did not differ (P > 0.05) in any other measured parameters of the plant, fruit quality, or yield. However, the total seasonal fruit yield was 13 to 31% higher and the total marketable fruit yield was 10 to 36% higher in various treatment programs compared to the standard program. The seasonal total of unmarketable fruit yield was also 4 to 25% higher in treatment programs than the standard program except that there were nearly 12% fewer unmarketable berries in the Actagro program compared to the standard program.
While treatments did not statistically differ for many of the measured parameters, numerical differences in marketable fruit yield could be helpful for some understanding of the potential of these biostimulants. Additional studies with larger treatment plots would be useful for generating additional data.
Acknowledgments
Thanks to Dr. Jenita Thinakaran for the assistance at the start of the study, Hamza Khairi for his technical assistance throughout the study, the field staff at the Shafter Research Station for the crop maintenance, NorCal Nursery for the strawberry transplants, and Actagro, BioGro, Locus, and Terramera for their collaboration and financial support
References
Dara, S. K. 2019a. Improving tomato yield with nutrient materials containing microbial and botanical biostimulants. eJournal of Entomology and Biologicals, 6 June 2019 https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=30448
Dara, S. K. 2019b. Evaluating the efficacy of anti-stress supplements on strawberry yield and quality. eJournal of Entomology and Biologicals, 10 August 2019 https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=31044
Dara, S. K. and D. Peck. 2018. Microbial and bioactive soil amendments for improving strawberry crop growth, health, and fruit yields: a 2017-2018 study eJournal of Entomology and Biologicals, 3 August 2018 https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=27891
Dara, S. K. and E. Lewis. 2018. Impact of nutrient and biostimulant materials on tomato crop health and yield. eJournal of Entomology and Biologicals, 9 January 2019 https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=26054
- Author: Surendra K. Dara
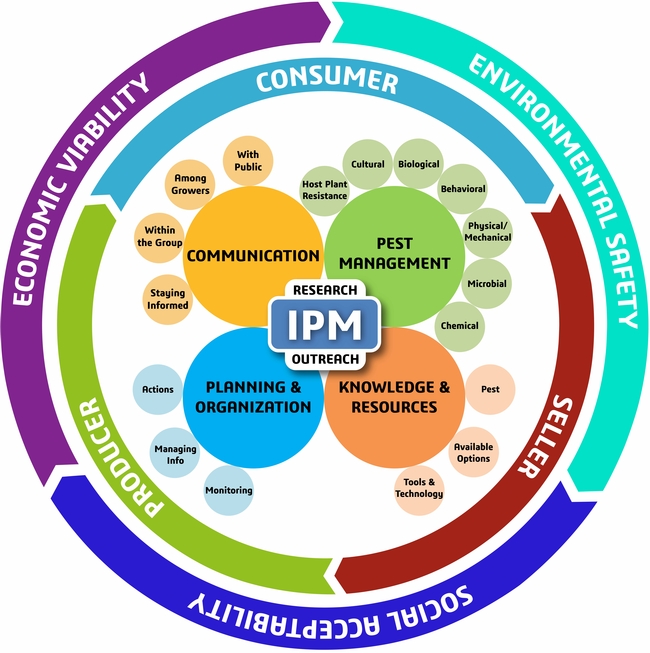
The traditional Integrated Pest Management (IPM) model is focused on maintaining ecological balance in the cropping system with some attention to the economics of pest management related to the yield losses. The new model, recently published in the Journal of Integrated Pest Management, is more comprehensive covering the management, business, and sustainability aspects of pest management and discusses various components within (Dara, 2019). IPM, according to the new model, can be defined as an approach to managing pests in an economically viable, socially acceptable, and environmentally safe manner.
New IPM model from Dara, 2019
Based on the information generated by several studies in California and other reports, here is how the new IPM model can be adapted for producing strawberries sustainably.
1. MANAGEMENT ASPECT
A. Pest Management: The term “pest” includes arthropod pests, diseases, and weeds and the management includes the various practices used to suppress them.
- Select varieties that produce good yields while resisting biotic and abiotic stresses.
- Choosing the right mulch and good irrigation and nutrient management contribute to good plant growth and health. Micro-sprinklers save water and hold pest management benefits.
- Explore the potential of beneficial microbes and biostimulants to improve nutrient and water absorption and to maintain crop health. Inoculate the transplants with biostimulants to induce systemic resistance and periodically apply, especially after fumigation, to improve the beneficial microbial activity in the soil.
- Healthy plants resist pest problems and reduce the need for control options. Plant health can be maintained through good cultural practices (biostimulants, nutrients, irrigation, soil conditioning, etc.).
- Predatory mites effectively control twospotted and Lewis mites, but natural enemy populations may not be sufficient to control the western tarnished plant bug.
- Light traps can be useful for managing lepidopteran pests.
- Tractor-mounted vacuums can be a part of the IPM program for managing the western tarnished plant bug, but their pest control efficiency is not necessarily superior to other strategies and are not without some associated risks. For example, operation of vacuums requires fossil fuels and they are used at a much higher frequency than pesticide applications.
- Use botanical, microbial, and chemical pesticides in combination. Combinations can improve pest control efficacy and rotations reduce the risk of resistance development.
- Rotating strawberries with crops such as broccoli can reduce the severity of certain soilborne diseases.
B. Knowledge and Resources:
- Understand pest biology, vulnerable stages of the pest, and appropriate strategies for each pest, different life stages, season, and budget. For example, relying on natural enemies for the western tarnished plant bug control is not effective and can lead to higher pest damage.
- Accurately identify the issue through visual observation or laboratory diagnosis for proper corrective action.
- Try to explore modern technology to monitor crop health.
C. Planning and Organization:
- Regularly monitor crop health for early detection and prevention of potential pest problems. For example, thorough scouting to determine the level of western tarnished plant bug infestation is very important for making the treatment decision. Deformed fruit is not always an indicator for the treatment threshold as nearly 1/3 of the fruit deformity occurs from environmental and other causes not related to the western tarnished plant bug.
- Look for signs of pesticide resistance and use appropriate strategies to reduce the risk.
- Maintain records of pest occurrence, seasonal trends, strategies that worked, and all relevant information, to build institutional knowledge for future use.
- Take the right action at the right time.
D. Communication:
- Regularly attend extension events and read research updates. Choose or design practices that are ideal for your farm based on the research updates.
- Periodically provide training to all individuals on the farm who directly or indirectly contribute to good agriculture practices.
- Share good management practices with each other for area-wide improvement of crop production and pest management.
- Try to educate the public so that they make better choices when purchasing produce. For example, good IPM practices can be more sustainable than organically approved practices and well-informed consumers can make a choice among conventional, organic, or sustainably produced grains, fruits, and vegetables. Public education can also help to eliminate otherwise good produce that is discarded because of minor imperfections. In strawberry, fruit deformity is caused due to the feeding of the western tarnished plant bug, genetic factors, poor pollination, or very low temperatures. Although most of the deformed strawberries, especially those from insect damage, have equal quality as marketable strawberries, they are discarded because of their shape. If the consumer market can accept deformed strawberries that still have good taste and nutritional quality, it can significantly reduce the wastage and the amount of pesticides sprayed to control the western tarnished plant bug.
2. BUSINESS ASPECT
- A strong IPM program can help growers produce sustainably while ensuring profitability.
- Consumer choices depend on their knowledge of sustainable agriculture. When they understand that produce with an IPM or Sustainably Produced label is safe for human and environmental health, it will have a major impact on food production systems.
3. SUSTAINABILITY ASPECT
- The current interpretation or perception of sustainability does not reflect true sustainability in terms of environmental health, profitability, food security, social equality, and other elements. A good IPM model can address all these issues to ensure farm productivity, food affordability, and environmental safety.
RESEARCH and OUTREACH
- Research and outreach component is the foundation of IPM to identify pest issues, develop appropriate knowledge for their management, and effectively disseminate the related information. Supporting research and outreach efforts of universities and other entities is essential for continuing IPM.
References
In addition to the below references, there are several articles in this eJournal on crop production and protection topics related to strawberry.
- Download “Biology and management of spider mites in strawberry” in English and Spanish at http://ucanr.edu/spidermiteguide or scan the QR code. Information about different species of spider mites and predatory mites is available in this guide.
- Efficacy of botanical, chemical, and microbial pesticides on twospotted spider mite and their impact on predatory mites http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=18553
- Entomopathogenic fungi can endophytically colonize strawberry plants when applied to the soil and negatively impact twospotted spider mite infestations http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=16821
- How to detect resistance to miticides in twospotted spider mite populations and strategies to reduce the resistance development http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=22097
- Comparison between the twospotted spider mite and the Lewis mite http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=5771
- An overview of lygus bug biology, damage, and management in strawberries http://cesantabarbara.ucanr.edu/files/75473.pdf
- Lygus biology, monitoring, and management videos http://ucanr.edu/SDYouTube
- Fruit deformity in strawberry from lygus bug and other factors http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=19630
- Potential of a solar-powered UV light trap as a pest management option in strawberry http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=25307
- IPM tools for controlling western tarnished plant bug in strawberry https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=19641
- Entomopathogens (pathogens of insects, mites, and ticks), their modes of infection, and how they can be used as a powerful tool in IPM http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=24119
- Biopesticides and IPM https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=25912
- Lygus bug and natural enemy populations in organic and conventional strawberries https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=14030
- Microbial and bioactive soil amendments for improving strawberry health and yields (2017-2018 study) https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=27891
- Beneficial microbe-based products for strawberry health and yield (2016-2017 study)
https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=25122
- Beneficial microbes and entomopathogenic fungi for strawberry health and yield (2015-2016 study) https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=22709
- Entomopathogenic fungi antagonizing Macrophomina phaseolina https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=28274
- Entomopathogenic fungi and other biologicals against Fusarium oxysporum
https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=22199
- Micro-sprinklers in strawberry https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=19699