
- Author: Mark Bolda
The element sulfur (S) has a large role in the management of plant disease. Growers are familiar with the biocidal formulations of sulfur, being elemental sulfur, sulfides, thiosulfate and fumigants like dimethyldisulfide (DMDS) and of course then we have sulfur dioxide which has been used as a postharvest preservative for dried fruits and vegetables.
However, beyond these outright biocidal effects we get from use of sulfur as a fungicide, there is also significant literature concerning the indirect effects of sulfur nutrition on reinforcing a plant's ability to inhibit and resist disease. Known as “Sulfur Induced Resistance” this is how one should frame the role of sulfur incorporated as a nutrient in plant response to disease.
There could be something to this. Work has been done showing that higher rates of S fertility affected infection rates and severity of fungal disease in oilseed rape and stem canker of potato. While informative, it is significant that the results of the former were achieved by sulfur additions to a field that was deficient.
How would sulfur induce resistance or inhibit disease in a plant? Sulfur goes to many places, including the amino acids acids cysteine and methionine, which are in 99% of proteins found in a plant. Findings cited by the chapter report that sulfur deficiency in the plant result in lower protein bound cysteine and free cysteine, which as the precursor to all relevant sulfur containing metabolites must have something to do with the ability to resist or inhibit disease.
Sulfur also goes to non-protein reservoirs in the plant, one of the main ones being glutathione. Glutathione, known as a phytoalexin because it is not formed prior to disease incursion, nevertheless accumulates rapidly after pathogen attack. It is involved in detoxifying signals necessary for fungal growth and could also be serving as a messenger to carry information to yet unaffected plant cells.
Phytoanticipins, in contrast to phytoalexins, are molecules in the plant which are preformed antibiotics- i.e. the plant produces them whether or not there is disease. Glucosinolates (of which our well known isothiocyanates are a cleaved product), on which sulfur plant nutrition has a strong influence, are one of these antibiotic phytoanticipins. Interestingly, low concentrations of glucosinolates don't necessarily equate with higher disease susceptibility, making them more of a qualitative defense for the plant.
Bottom line: The role of sulfur in disease resistance and inhibition in plants is a very important one, but it's a pretty sure thing that these systems function perfectly well in sulfur sufficient soils, which describe pretty well every one in the Pajaro and Salinas Valleys. As such, while the value of sulfur as a foliar fungicide is indisputable for certain diseases, I am not seeing the value of pursuing sulfur work experimentally as a soil disease mitigant.
The above is a summary of some of the aspects of Chapter 8: Sulfur in Plant Disease from “Mineral Nutrition and Plant Disease” edited by Lawrence Datnoff, Wade Elmer and Don Huber.
- Author: Mark Bolda
- Author: Tom Bottoms
- Author: Tim Hartz
It has been more than 30 years since UC published strawberry leaf nutrient diagnostic guidelines (Publication 4098, ‘Strawberry deficiency symptoms: a visual and plant analysis guide to fertilization’, released in 1980). In the years since that publication, varieties, production practices and yield expectations have changed considerably. In 2010 we began a project, funded by the California Strawberry Commission, to reevaluate leaf and petiole nutrient sufficiency ranges for day-neutral strawberries. With the cooperation of many berry growers in the Watsonville-Salinas and Santa Maria areas we collected leaf and petiole samples from more than 50 ‘Albion’ fields over the past two production seasons. In each field samples were collected 5 times over the production season, from early spring through September, to document the nutrient concentration trends from pre-fruiting to post-peak production. Leaf samples were analyzed for total concentration of nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), zinc (Zn), manganese (Mn), iron (Fe) and copper (Cu). Petioles were analyzed for NO3-N, PO4-P and K concentration.
After the season cooperating growers provided yield information, which allowed us to categorize the fields as being ‘high yield’ or 'low yield’. We then applied a process called DRIS (Diagnosis and Recommendation Integrated System) to mathematically evaluate the difference in nutrient concentrations as well as nutrient ratios between high yield and low yield fields. This process allowed us to identify which of the high yield fields were ideally balanced nutritionally. From this group of nutritionally balanced, high yield fields we were able to calculate a DRIS sufficiency range for each nutrient at each growth stage.
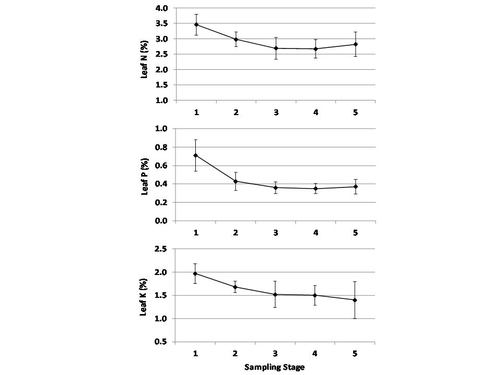
Fig. 1 shows that leaf N, P and K concentrations were highest before harvest began (stage 1, which was late February in Santa Maria and late March in Watsonville-Salinas), and declined to a reasonably stable level throughout the main harvest period (stages 3-5, May-July in Santa Maria, June-August in Watsonville-Salinas). The decline in leaf macronutrient concentrations during the peak harvest period was expected; it happens in many fruiting crops because the leaves rapidly translocate nutrients to the developing fruit. By contrast, micronutrient concentrations either increased from early vegetative growth to the main harvest period (as was the case for B, Ca and Fe), or remained reasonably stable over the entire season (all other micronutrients). The vertical bars on each data point on Fig. 1 indicate the range of values typical of nutritionally balanced, high yield fields at each growth stage. These are the DRIS sufficiency ranges; leaf nutrient concentrations within these ranges can safely be assumed to be adequate for high yield production.
Table 1 lists the DRIS leaf nutrient sufficiency ranges for pre-harvest and main harvest growth stages. For the sake of comparison, both the sufficiency ranges given in UC Publication 4098 and the current University of Florida guidelines have been included. Although for most nutrients the ranges match pretty well, for others there are substantial differences. Where the DRIS sufficiency range is substantially higher than the other sources (Ca, Mn and Fe, for example) it is because those nutrients are in such abundant supply in our coastal soils that plant uptake is far in excess of actual plant requirement; for those nutrients a lab test result marginally below the DRIS range would not be a matter of concern.
For several nutrients (N, Zn and Cu) the DRIS sufficiency range fell below the other recommendations. We are confident that the DRIS ranges represent nutrient sufficiency because they were determined by measuring the levels common in high yield fields. The field survey approach used in this project ensured that a wide range of field conditions and grower practices were included, so the results are broadly representative of the coastal industry. Also, for all three nutrients the average leaf concentrations of the high yield and low yield groups were essentially equal, suggesting that availability of these nutrients did not limit yields.
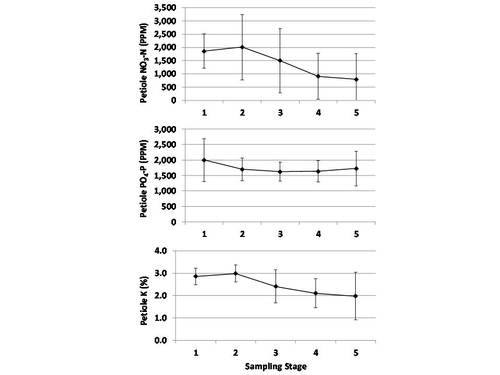
Fig. 2 shows the trends in petiole nutrient concentrations over the season. Petiole NO3-N was so highly variable as to be nearly worthless as a diagnostic technique; during peak fruit harvest (our sampling dates 3 and 4) petiole NO3-N in high yield fields varied from < 200 PPM to 2,600 PPM. While we believe that leaf total N is a more reliable measurement, this study suggests that maintaining petiole NO3-N > 1,000 PPM pre-harvest, and > 400 PPM during peak harvest, is adequate to maintain high productivity. Given the high variability of petiole NO3-N it is possible that concentrations < 400 PPM would be adequate during the summer.
Petiole PO4-P and K were less variable than petiole NO3-N. Maintaining PO4-P > 1,200 PPM throughout the season should ensure P sufficiency. Given the high soil P availability in most coastal soils rotated with vegetable crops, this level is probably much higher than the ‘critical value’. Maintaining petiole K > 2.5% preharvest, and > 1.5% during peak harvest, appears to be adequate.
Table 1. Comparison of DRIS leaf nutrient sufficiency ranges with prior UC recommendations, and current University of Florida guidelines.
|
|
|
Nutrient sufficiency ranges |
||
|
Growth stage |
Nutrient |
DRIS |
UC Pub. 4098 |
University of Florida |
|
Pre-harvest |
% N |
3.1 - 3.8 |
|
3.0 - 3.5 |
|
|
% P |
0.50 - 0.90 |
|
0.20 - 0.40 |
|
|
% K |
1.8 - 2.2 |
|
1.5 - 2.5 |
|
|
% Ca |
0.6 - 1.3 |
|
0.4 - 1.5 |
|
|
% Mg |
0.33 - 0.45 |
|
0.25 - 0.50 |
|
|
% S |
0.19 - 0.23 |
|
0.25 - 0.80 |
|
|
PPM B |
31 - 46 |
|
20 - 40 |
|
|
PPM Zn |
13 - 28 |
|
20 - 40 |
|
|
PPM Mn |
75 - 600 |
|
30 - 100 |
|
|
PPM Fe |
70 - 140 |
|
50 - 100 |
|
|
PPM Cu |
3.3 - 5.8 |
|
5 - 10 |
|
|
|
|
|
|
|
Main harvest |
% N |
2.4 - 3.0 |
> 3.0 |
2.8 - 3.0 |
|
|
% P |
0.30 - 0.40 |
0.15 - 1.30 |
0.20 - 0.40 |
|
|
% K |
1.3 - 1.8 |
1.0 - 6.0 |
1.1 - 2.5 |
|
|
% Ca |
1.0 - 2.2 |
0.4 - 2.7 |
0.4 - 1.5 |
|
|
% Mg |
0.28 - 0.42 |
0.3 - 0.7 |
0.20 - 0.40 |
|
|
% S |
0.15 - 0.21 |
> 0.10 |
0.25 - 0.80 |
|
|
PPM B |
40 - 70 |
35 - 200 |
20 - 40 |
|
|
PPM Zn |
11 - 20 |
20 - 50 |
20 - 40 |
|
|
PPM Mn |
65 - 320 |
30 - 700 |
25 - 100 |
|
|
PPM Fe |
85 - 200 |
50 - 3,000 |
50 - 100 |
|
|
PPM Cu |
2.6 - 4.9 |
3 - 30 |
5 - 10 |
- Author: Mark Bolda
by Steven Koike
UC Cooperative Extension
Starting around mid-May and extending into June, strawberry growers and pest control advisors in coastal California are observing the fruit problem known as bronzing. This problem occurs every year to some extent but can result in large economic losses in some seasons. Bronzing results in a tan or bronzed discoloration on green and ripening strawberry fruit. Bronzed fruit have dried, rough surfaces that render the fruit unmarketable (Photos 1 and 2). The skin of such fruit can later crack.
There are three types of bronzing. Type I bronzing occurs on distinct, localized parts of the fruit, often beneath the fruit calyx or around the strawberry seeds (achenes), and is caused by insect feeding, primarily thrips (physical damage due to abrasion (Photo 3) is also very localized). Type II bronzing is caused by chemical sprays that cause a one-sided bronzing to the side of the fruit exposed to the application. In contrast, Type III bronzing covers virtually the entire surface of the fruit, occurs during certain periods of time, and can result in devastating crop loss. It is notable that Type III bronzing in coastal California tends to occur when the weather is sunny and warm.
Field-based research has demonstrated that Type III bronzing is associated with fruit exposure to stressful environmental conditions that include extreme solar radiation, high temperatures, and low relative humidity. This Type III problem is not caused by sulfur applications or feeding by thrips, mites, or other pests.
The problem is difficult to manage and prevent. Strawberry cultivars differ in their susceptibility to Type III bronzing, so growers should consider this factor when selecting cultivars for planting. Growing the strawberry crop so as to reduce physiological stress to the plants is a general overall recommendation. Observant growers and PCAs noted that commercial fields that happened to receive insecticide or fungicide sprays prior to a high temperature, high-sunlight intensity bronzing period had in many cases significantly lower bronzing losses compared to adjacent untreated fields. This situation likely occurred because commercial pesticides usually contain additives that protect the products from solar and ultraviolet radiation; such sprays also provided similar protection to the strawberry fruit.




