Posts Tagged: lettuce
Lawn-pocalypse! Surviving Drought
Ah, summer! The season of sunburns, pool parties, and… lawn droughts. If your once lush, green carpet now looks like a crunchy brown doormat, you're not alone. Let's dive into why your yard is staging a dramatic death scene and what you can do to...

Bermuda grass and weeds overtaking drought stressed turf grass.
Predators by drone: Biological control research
Two of the worst pests plaguing lettuce growers in the Salinas Valley area are aphids, specifically lettuce-currant aphids (Nasovonia ribisnigri), and western flower thrips (Frankliniella occidentalis). Lettuce-currant aphid is an invasive pest that sets up shop in the heart of the lettuce plant and will render the crop unsellable when it reaches high enough numbers. Thrips can both cause cosmetic damage to lettuce crops and are also responsible for the spread of Salinas impatiens necrotic spot virus (INSV), the fatal lettuce disease that has driven large losses since the 2020 growing season.
While effective tools exist to control both aphids and thrips, they are almost exclusively chemical. Chemical sprays are increasingly under pressure due to changes in the regulatory framework in California as well as the development of pest resistance and discoveries of key chemistries in area watersheds1,2. The UC Davis FiVE lab biological control research program addresses a growing interest in developing alternative tools for managing both pests that do not rely on chemical applications. Biological control provides an opportunity for the management of thrips and aphids that do not rely on chemical tools.
Biological control is defined as the use of natural enemies to control a target pest. Three general categories of biological control could possibly be used as management practices for lettuce pests in the Salinas Valley area:
• Conservation biological control refers to the establishment and maintenance of resources and conditions favorable
to a native or endemic beneficial species. Instead of releasing predators into crop fields, specific types of flowers and other habitats are planted to attract beneficial species that are already a part of the local ecosystem. To date, most efforts on biological control in lettuce have used the conservation biological control approach.• Inundative biological control involves the release of a beneficial insect species in large numbers with the expectation that the beneficials that are released will only provide control for a short amount of time before eventually dying out. Such releases would need to be repeated at regular intervals for the duration of the growing cycle for a crop.
• Augmentative biological control refers to the use of releases of smaller numbers of beneficials to areas where a smaller population of the species already exists, but not in numbers great enough to provide adequate control of the targeted pest species. The goal of augmentative releases is to bolster already-existent populations of beneficial species so they achieve great enough numbers to provide control of the pest or pests of interest.
Conservation biological control in the Salinas Valley
Syrphid flies
Aphid pests of lettuce have been effectively managed in some lettuce production systems through the planting of sweet alyssum adjacent to and interspersed within crop fields3. Sweet alyssum is a favorite of the Syrphid fly (Diptera: Syrphidae), the primary biological control agent used to control aphid pests in lettuce. Syrphids, also called hoverflies or flower flies, are a family of black and yellow pigmented flies which resemble bees and stinging wasps. The coloration is a protective camouflage; Syrphid flies are harmless to humans. Syrphid adults are frequently seen visiting flowers for their nectar and pollen, which the insect consumes both as an energy source and to support their reproduction.
In exchange the female Syrphid flies will lay eggs in lettuce plants with lettuce aphid infestations, the primary food source for their young. Once the eggs hatch, the syrphid maggots, which are predatory on slow, soft-bodied insects, will feed on the aphids and suppress their population. Syrphid larvae are known to be voracious; some California species have been shown to consume upwards of 100 aphids per day4!
Syrphids are the intended beneficiaries of most conservation biological control in central coast lettuce fields, but other beneficial species take advantage of these resources as well.
Other predatory species love sweet alyssum
Many other biological control agents are supported by insectary plantings5. Ladybird beetles often inhabit lettuce fields and may provide some control of lettuce aphid infestations. Common lacewings (family Chrysopidae) are also found in lettuce fields and insectary plantings. Lacewings, which are only predatory in their immature or larval life stage, can provide biological control services against lettuce aphids and western flower thrips. Minute pirate bug (Orius sp.) and aphid midges (Aphidoletes aphidimyza) have also been observed in and collected from insectary plantings in lettuce fields, but it is not known the extent to which they can suppress populations of lettuce aphid or Western flower thrips.
UC Davis Fi-VE Bug IPM Lab biological control research programs
Including insectary plantings to attract naturally occurring predators has historically been the only efficient way to get beneficial species into crop fields. Newly developed technology using drones as a dispersal tool may provide another option for growers interested in using biological control as part of their pest management programs for aphids and thrips. This technology drastically reduces the time and labor required to conduct large releases of laboratory-reared beneficial insects, making the approach more feasible for growers.
As part of a research program funded by the California Department of Pesticide Regulation (CA DPR) and in collaboration with Daniel Hasegawa at USDA-ARS and with Parabug, we are studying the release of biological control agents using drones for the management of aphid and thrips pests of lettuce crops. Our three experimental programs are as follows:
In-field inundative releases of green lacewing larvae and predatory cucumeris mites to control aphids and thrips in lettuce
Experiments run by former Monterey County IPM Advisor Alejandro Del Pozo-Valdivia found that a single inundative release of green lacewing eggs (Chrysoperla rufilabris) in lettuce fields reduced aphid pressure six weeks after release6. Our experiment builds on Alejandro's work, examining whether repeated releases of green lacewing eggs throughout the lettuce growing cycle reduce aphid numbers. Additionally, the experiment includes two treatments aimed at suppressing western flower thrips: inundative releases of a species of predatory mite (Amblyseius cucumeris), and a combined release of both predatory mites and green lacewing eggs.
Augmentative releases to bolster non-syrphid predatory species in insectary strips and intercropped alyssum
Other native predators of aphids and thrips are present in the insectary plantings growers use to attract syrphids, but their numbers are too low to provide suppression of thrips and aphids in adjacent crops. These species are reared by commercial insectaries, but using them in an inundative release could prove too costly for growers. Experiments in this program examine the use of smaller releases of these predatory species early in the growing cycle over insectary plantings. The goal is to determine whether the presence of floral resources allows the predators to stick around and build up enough in population to control aphids and thrips in the crop field. Experiments will be conducted with aphid midge (Aphidoletes aphidimyza), an aphid predator, and minute pirate bug (Orius insidiosus), a predator of western flower thrips.
Augmentative releases to manage thrips in non-crop areas
Western flower thrips plague not just vegetable crop fields but also the vegetation surrounding crop areas. In this experiment, we will examine whether releases of cucumeris mites and minute pirate bugs over field edges planted with ice plant will establish these predators in the vegetation and provide long-term suppression of western flower thrips.
Citations
- Deng, X. Study 321: Surface water monitoring for pesticides in agricultural areas in the Central Coast and southern California (2022)
- Gao, Y., Lei, Z. & Reitz, S. R. Western flower thrips resistance to insecticides: detection, mechanisms and management strategies. Pest Manag. Sci. 68, 1111–1121 (2012).
- Brennan, E. B. Agronomic aspects of strip intercropping lettuce with alyssum for biological control of aphids. Biol. Control 65, 302–311 (2013).
- Hopper, J. V., Nelson, E. H., Daane, K. M. & Mills, N. J. Growth, development and consumption by four syrphid species associated with the lettuce aphid, Nasonovia ribisnigri, in California. Biol. Control 58, 271–276 (2011).
- Bugg, R. L., Colfer, R. G., Chaney, W. E., Smith, H. A. & Cannon, J. Flower Flies (Syrphidae) and Other Biological Control Agents for Aphids in Vegetable Crops. (University of California, Agriculture and Natural Resources, 2008). doi:10.3733/ucanr.8285.
- Del Pozo-Valdivia, A. I., Morgan, E. & Bennett, C. In-Field Evaluation of Drone-Released Lacewings for Aphid Control in California Organic Lettuce. J. Econ. Entomol. 114, 1882–1888 (2021).
Growing Lettuce
Lettuce may not be a garden crop that causes you to perk up and say, “Tell me more.” You may be thinking, “Boring, I'll pass.” Or you may be the gardener who says, “I never could get that crop to grow.” Whatever your current thinking about lettuce, I hope to get you excited about this humble crop. There are not many garden vegetables with more diversity than lettuce, with more than 100 varieties in a wide range of colors, textures, and shapes.
If you need more encouragement, lettuce is a great hydrator, being 95% water. Lettuce is a powerhouse when it comes to vitamins and minerals. Vitamins A and C play a strong role in supporting the immune system, eyesight, and reduce inflammation. Iron, folic acid, calcium and potassium also add to the health benefits of lettuce.
Lettuce (Lactuca sativa) has been cultivated for thousands of years. The name is derived from the milky liquid produced when a leaf is broken from the stem.
Cool Season vs. Warm Season
We tend to think of lettuce as a light summer food, but lettuce is a cool weather crop. The optimum growing conditions for lettuce occur in fall and early spring. Many cool season lettuce varieties have good tolerance for cold temperatures and do well in low-light conditions.
Summer lettuce is in high demand as a crop, causing breeders to develop lettuce varieties for summer growing conditions with good success. These lettuce varieties are bred specifically to be heat tolerant and are slow to bolt and less bitter as they mature.
Seed Germination & Transplants
Lettuce seeds will germinate in 3–10 days depending on conditions.
Seeds can germinate in soils as low as 40°F but germination will be slow. The optimum temperature for seed germination is 55°–65°F. Above 70°F lettuce seeds germinate poorly, resulting in undersized, misshapen, elongated heads. At higher temperatures lettuce seeds may completely fail to germinate.
Fall lettuce seeds need to be in the ground in late September so they can reach 75% maturity going into the low-light days and cool nights of our winter months.
Spring lettuce seeds should be started indoors in early February. This will give you a jump on planting out in the spring after all danger of frost is past. Continue to sow seeds at 2-week intervals for continuous production.
Lettuce seeds are very small and should be planted with a very light covering of soil (¼ inch). Be generous when sowing your seeds, as some will not germinate, and others may be lost to birds. As they mature, the thinnings can be added to salad or used in sandwiches. The final spacing for lettuce plants should be 9–12 inches.
Many gardeners start with lettuce transplants from a local nursery, followed by seed sowing at 2-week intervals. This gets you off to a quick start and keeps you in a continuous supply of lettuce. To keep your lettuce crop growing through the summer switch to a variety listed as heat tolerant or slow-to-bolt.
Soil Preparation
Lettuce does best in loose, well-drained soil with a generous addition of compost added before planting. A source of organic nitrogen can also be added to the soil before planting. Alfalfa meal, cottonseed meal, and fish or kelp meal are all good sources for slow, consistent nitrogen release.
Watering & Fertilizing
Keep the top 1–3 inches of the soil consistently moist as lettuce roots are shallow and dry out easily. You will want to provide adequate nitrogen for this fast-growing crop. Organic fertilizer is slower to become available. If you are using transplants, give them an application of liquid fertilizer 2 weeks after transplanting.
Frost Protection & Shade Cloth
Winter grown lettuce, though it can tolerate cool temperatures, will need protection from frost, while summer grown lettuce must have some shade protection.
Harvesting
Many lettuces can be harvested as baby greens. Some of the looseleaf varieties can be harvested in any stage of development by cutting the larger outside leaves and leaving the smaller inner leaves to mature. Mature lettuce does not get better, it gets bitter, so don't wait to harvest. To keep lettuce at peak freshness, harvest early in the morning. Wash the leaves thoroughly in cold water, then remove as much excess moisture as you can—a salad spinner is a great tool for this job. Store lettuce in the refrigerator in a plastic container with a lid with several damp paper towels added to create a humid environment. Garden fresh lettuce, stored in this manner, will last 7–14 days.
Disease & Pests
The most common diseases of lettuce are Botrytis rot, lettuce mosaic virus, and mildew. Keeping lettuce leaves dry is the best way to avoid disease from getting a foothold. Good air circulation and drip irrigation are two helpful practices.
Lettuce may fall prey to occasional pests including cutworms, leafminers, caterpillars, aphids, whitefly, snails/slugs and earwigs. Access the UC IPM website for information on controlling specific disease, insects, and vertebrate pests such as birds and deer.
https://ipm.ucanr.edu/home-and-landscape/lettuce/index.html
Lettuce Types
Butterhead / Boston / Bibb: These lettuce varieties form a round, loose head. They range from delicate and buttery to bright and crisp. Leaves tend to be large, making them an excellent choice for wraps and sandwiches. *Days to maturity 45–55. These varieties can be harvested anytime during development.
Looseleaf: This lettuce grows from a central stalk but does not form a head. These varieties are easy to grow and perform well in shaded areas or in low light. They are tolerant of warm temperatures and are slow to bolt which make them a good choice for multi-season growing. *Days to maturity 45-55. These varieties can be harvested anytime during development.
Crisphead / Summercrisp / Batavian: These lettuce varieties are known for their crisp snap and sweet flavor. They form a round, compact head. Often the outer leaves are darker with tender light leaves in the interior. Crisphead is a true cool weather variety which will stand up to very cool temperatures but beware, it is quick to bolt when temperatures rise and it's a favorite of snails and slugs. This would be a good choice for starting indoors in January for transplanting in February after all danger of frost is past. *You will need to plan ahead as some of these varieties take 70-100 days to reach full maturity.
Cos / Romaine: This lettuce is very upright, forming a column. They can be tightly closed, semi-closed, or open heads. This lettuce has a crisp, creamy white heart with the outer leaves remaining sturdy and dark. It is unforgiving of poor growing conditions and is not well adapted to warm weather. There are interesting varieties in the seed catalogs with some beautiful deep burgundy varieties in the offering. *You will need to plan ahead as some of these varieties take 75-85 days to reach full maturity.
I hope I have piqued your interest in experimenting with both winter and summer lettuce in your garden. The varieties and variations are truly exciting, many of which you will never see in your supermarket or even at farmers markets. Whether you select your varieties by color, shape, texture, or even their fun names, I hope you have decided to give lettuce a place in your garden. May it be a delightful and successful experience!
Help Desk of UC Master Gardener Program of Contra Costa County (BHD)
Research Update: Thrips migration from the hills?
“In the coastal areas where spotted wilt is a serious problem ... there is much to be learned concerning the seasonal migrations and local host succession of the thrips”
At first, this looks like a quote from 2020 or 2022 made in reference to the recent outbreaks of Impaciens necrotic spot virus (INSV), a thrips-transmitted disease currently affecting lettuce. But the quote is actually pulled from a UC publication titled Thrips of economic importance in California, authored by Professor Stanley Bailey in 1938.
The lettuce industry here in the Salinas Valley has been hit hard by INSV the past few years, and understanding the biology of the thrips that vectors the virus could be critical for management. But as Bailey noted almost 100 years ago, fully understanding the biology of western flower thrips has been elusive for decades.
In Thrips of economic importance in California, Bailey also noted that thrips in coastal areas tend to spend their summers at higher altitudes, but as native hosts dry up, they can concentrate on nearby crops. I wondered if such a migration could help explain the increase in thrips and INSV pressure the past few falls. With advances in thrips collection methods (i.e., sticky cards) and changes in cropping patterns, I was curious -- could we could observe the migration that Bailey described nearly 100 years ago?
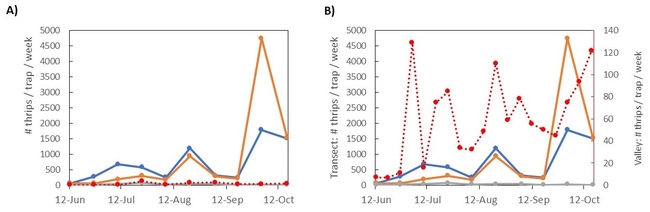
Methods: With help from John Massa (Comgro) and a team from Braga Fresh (Eric Morgan, Katie Chiapuzio, and Jaylen Calabro), I set up a loose transect of 10 sticky card traps at about 4' off the ground (Figure 1). The transect spanned 0.38 miles (610 m) and an elevation change of 325 ft (99 m).
The first traps were deployed on June 5th and the last traps were collected on October 25th. We swapped out cards every two weeks for a total of 10 sets of cards. Some cows used two of the lower traps as scratching posts, so we were limited to 8 traps for most of the trial.
Sticky cards were taken back to the lab to count any thrips that fit the general description of Western flower thrips, Frankliniella occidentalis: less than 2 mm long, overall yellow to brown body color. Some larger, black thrips were occasionally found on traps and were excluded from overall counts.
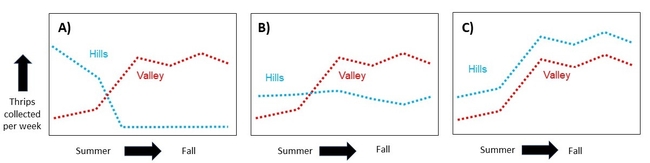
Hypothesis: Thrips migrate down from the hills in late summer and early fall, increasing the thrips pressure in the valley which could increase the risk of spreading INSV.
Expected Results: If thrips counts are high in the hills in summer, but drop as populations rise in the valley, then this would be good support for Bailey's note and my hypothesis (Figure 2A). Alternatively, if thrips populations in the hills are consistent across time (Figure 2B), or if their population fluctuations match what is going on in the valley (Figure 2C), then it is unlikely that a mass migration is occurring.
Results: The transect results are summarized in figure 3. In panel A, the average thrips per week is plotted over time, with cards grouped by location (top of the hill, middle of the hill, or towards the bottom). The bottom traps were mostly surrounded by dried grass, while the top and middle traps were generally near chaparral plants that stayed green and flowering throughout the summer and into the fall. You can see an increase in thrips captures from June into July, followed by a dip in early august, and two more peaks in mid-August and early October (following that three-day heatwave). Compared to the valley counts (red line), the number of thrips captured on the hill was much higher, an average of 13 times higher than in the Valley. Adjusting the scale of the Valley-level trap counts (Figure 3B), we can see the Valley traps somewhat followed a similar pattern - thrips populations peaked in early June, had a few weeks of low counts in early August, then peaked again in mid-August and early October. With some variation, adult thrips captures in the hills followed a similar pattern to those captured nearby in the Valley.
Preliminary Conclusions: Contrary to my hypothesis, this small study does not provide evidence that thrips migrate en masse from the hills into the Salinas Valley. The hills maintained some green vegetation and flowers throughout the year, so thrips may not be driven to the Valley like Bailey described. Instead, the hills supported high thrips population throughout the summer and into the fall, which may have acted more like a continuous source of thrips into the valley. This could have interesting effects on INSV epidemiology, depending on whether the host plants in the hills can acquire INSV.
We of course cannot rely on a single transect in one year to conclude that thrips never migrate en mass into the Valley. This year we had an atypical, cool, wet spring that may have changed if or how thrips migrate. Perhaps migration only occurs in years with a drier, warmer spring. We also cannot discount the fact that the thrips we counted may not all be Western flower thrips; the identification characteristics we used (less than 2 mm long, overall yellow to brown body color) are not diagnostic of Western flower thrips. The next steps in this study would be to set up additional transects next year and live collection of thrips off of vegetation. By setting up additional transects (and getting them set up earlier in the season), we could determine if this preliminary transect was an anomaly, or if thrips are not behaving the way that Bailey described in 1938. Live collection of thrips is necessary to determine what proportion of thrips in the hills are Western flower thrips that can vector INSV. Either way, we are one step closer to understanding the seasonal migrations and local host succession of thrips, which could help us in our fight against INSV.
Much thanks to John Massa, Eric Morgan, Katie Chiapuzio, Jaylen Calabro, Jasmine Rodriguez, Luis Ramirez-Espinoza, and Carlos Rodriguez Lopez!
Research Update: Sampling for INSV+ thrips in vegetable transplants
We are getting to the time of year where lettuce production winds down in the Salinas Valley and ramps up in the desert around Yuma, Arizona. Unlike in the Salinas Valley, the desert has not been hit as hard by Impaciens necrotic spot virus (INSV), the virus that is transmitted to lettuce by Western flower thrips (F. occidentalis). When the virus does show up in the desert, the primary infection can often be traced back to INSV-infected thrips that arrived on vegetable transplants from coastal California (Palumbo, 2022)
These finding stirred up concern in the local ag community – could vegetable transplants also be a significant source of new INSV infections in the Salinas Valley? There are instances where recently transplanted fields start showing INSV symptoms soon after planting (Figure 1), but it is challenging to nail down if they were infected before or after transplanting. After gaining some advice from John Palumbo of the University of Arizona (who has been spearheading the ISNV work in the desert), I set out to sample thrips from local transplant nurseries.
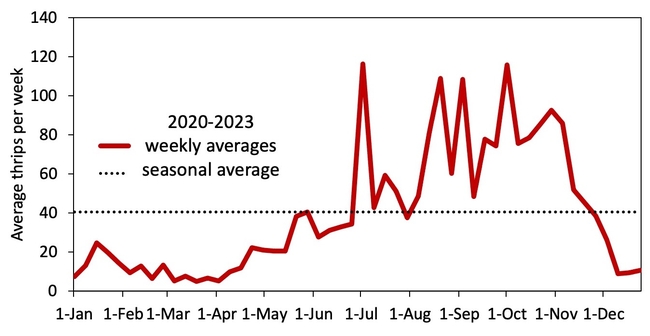
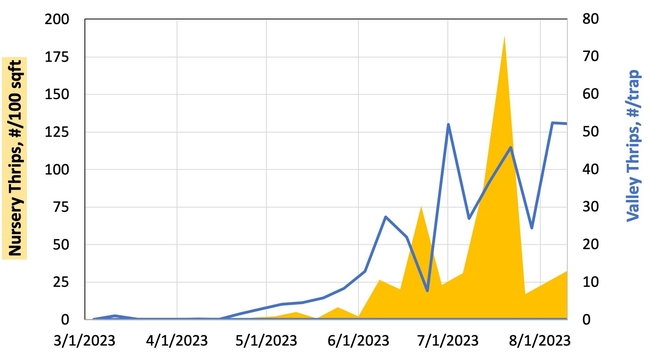
Hypothesis: Vegetable transplants are NOT a major source of INSV-infected thrips in the Salinas Valley. Although this hypothesis may seem contrary to the findings from Yuma, it all comes down to timing of transplant production and background levels of thrips and INSV. As summarized in Figure 2, weekly thrips activity is low in spring and early summer but high in late summer and early spring. [check out an interactive version of these data: Salinas Valley Lettuce Pest Mapping Tool]. This difference in activity somewhat corresponds to when transplants are grown for local use (spring and early summer) versus desert use (late summer and fall). Transplants that are sent to Yuma are grown when thrips populations and INSV pressure has been historically high – putting them at higher risk of carrying INSV+ thrips.
Species confirmation and virus testing: I collaborated with Daniel Hasegawa (USDA-ARS) for species confirmation and virus testing. Daniel and his team used a genetic testing method called multiplex RT-qPCR to determine if the adult and larval thrips were 1) Western flower thrips (F. occidentalis), 2) Onion thrips (Thrips tabaci), or 3) some other thrips species AND if the thrips were carrying 1) INSV or 2) Tomato spotted wilt virus (TSWV, similar to INSV). For small samples (five or fewer thrips), Daniel's team tested each thrips individually. For samples with more than five thrips, they tested half of the thrips as a pooled sample. If this sub-sample tested positive for INSV, a subset of the remaining thrips were tested individually.
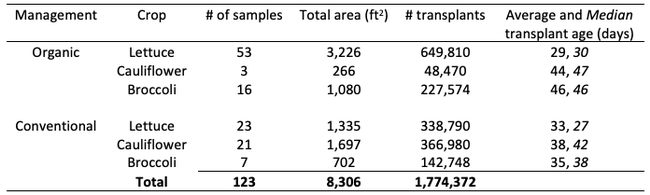
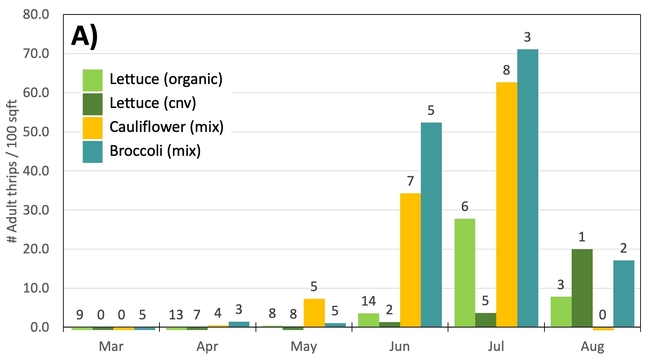
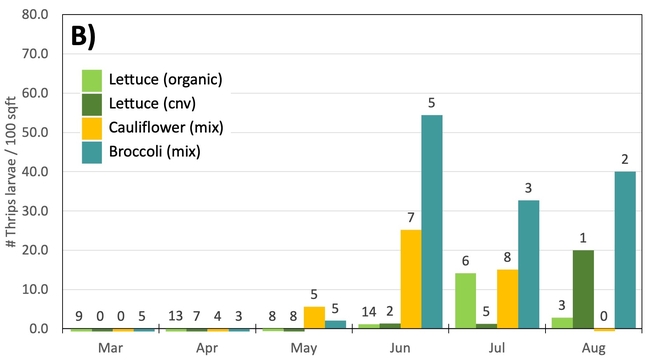
Results: From March through May, we only collected 52 thrips, 50 of which were identified as western flower thrips and none which tested positive for INSV or TSWV. In June, collection shot up almost 10-fold and doubled again in July, somewhat tracking the thrips population increase in the valley (Figure 4). In June and July, thrips densities were consistently higher on brassica transplants compared to lettuce transplants (Figure 5).
Despite collecting over 2,000 individual thrips (1,333 adults and 782 larvae), only two samples, or 1.64% of the tested thrips, tested positive for INSV. The two samples which has positive hits for INSV were collected in June, one off or organic broccoli and one off of conventional cauliflower. None of the thrips collected off of lettuce transplants tested positive for INSV.
Preliminary Conclusions: For the Salinas Valley, the thrips populations on transplants appear to mirror the thrips population in the valley. This is perhaps not too surprising since most transplants are grown on uncovered benches near crop fields. Despite lettuce being a better host for Western flowerthrips (Joseph & Koike, 2021), we consistently collected morethrips frombrassica transplants. We attribute this difference to management practices (e.g., insecticide applications on lettuce transplants) and because brassica transplants tend to stay at nurseries for an additional week.
With this in mind, we may expect that transplanted lettuce fields in the Salinas Valley are more likely to be infected by INSV+ thrips coming in from nearby areas (the prior crop, nearby fields, or weedy areas) rather than thrips from transplant nurseries. Although I would expect to find more INSV+ thrips on vegetable transplants in a year with higher INSV incidence (2023 incidence was considerably lower than prior years), the risk of INSV+ thrips coming in from other sources would increase as well.
For the desert, however, the background risk of INSV is much lower because INSV levels drop off over the summer. In this context, even a handful of INSV+ thrips on transplants pose a proportionally greater risk than in the Salinas Valley. Recognizing this risk to growing regions beyond the Salinas Valley, it is important to continue monitoring INSV levels in transplant nurseries and to work with nurseries to minimize the risk of transporting INSV+ thrips.
References:
- Palumbo, JC, 2022. Thrips and INSV Management in Desert Lettuce. University of Arizona VegIPM Update, Vol 13, No 22, Nov 2, 2022. https://acis.cals.arizona.edu/docs/default-source/agricultural-ipm-documents/vegetable-ipm-updates/2022/thrips-and-insv-management-in-desert-lettuce.pdf?sfvrsn=3088b8b9_2
- Joseph, SV, Koike, ST, 2021. Could Broccoli and Cauliflower Influence the Dispersal Dynamics of Western Flower Thrips (Thysanoptera: Thripidae) to Lettuce in the Salinas Valley of California? Environmental Entomology 50, 995–1005. https://doi.org/10.1093/ee/nvab050
Thanks to Daniel Hasegawa and the entire Hasegawa lab (USDA-ARS, Salinas); John Palumbo (University of Arizona); Kevin Costa, Thomas Costa, and Manuel Aguirre (Headstart Nursery); Francisco Castaneda and Omar Saenz (Growers Transplanting); Lupe Guillen, Maria Alfaro, Alejandro Palma-Carias, and Jim Wilkinson (Dole Fresh Vegetables); Jasmine Rodriguez; Luis Ramirez-Espinoza (CSU-MB); and the California Leafy Greens Research Board.