- Author: Surendra K. Dara
As the food production faces the persistent threat of endemic and invasive pests, researchers continue to develop new technologies and strategies for protecting crops from these threats. One such new technology is RNA interference (RNAi) with targeted mechanisms towards specific pests. RNAi can be used as a trait in a crop or as a sprayable product against the target pest. Before delving further into this here are a few basic details of this biological process that will help understand the RNAi mechanism.
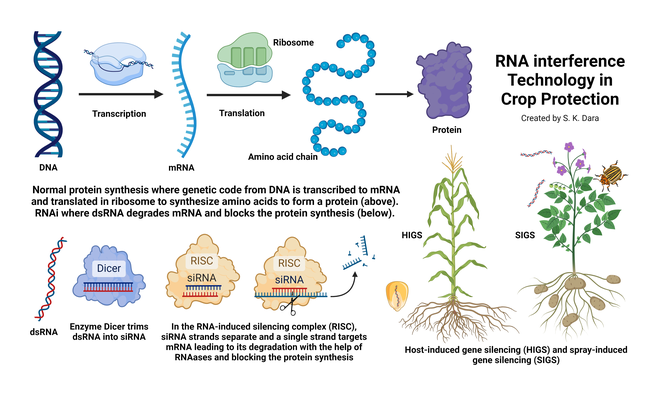
Deoxyribonucleic acid (DNA) in the chromosomes of most living organisms contains genetic code for making proteins that are essential for various biological processes. Ribonucleic acid (RNA) carries the genetic code from DNA to the protein-making factories within the cell known as ribosomes. DNA has two strands of nucleotides (sets of deoxyribose sugar with nitrogenous bases connected by a phosphate group) whereas RNA has only one strand of nucleotides. RNA also differs from DNA in having ribose sugar, instead of deoxyribose, and a different kind of nitrogenous base. The purpose of RNA is to transfer the genetic code from DNA as amino acids are made in ribosomes. A chain of amino acids makes a specific protein. Examples of proteins in insects include juvenile hormone responsible for development and reproductive maturation, ecdysone responsible for molting and metamorphosis, digestive enzymes like amylases, glycosidases, lipases, and proteases, and esterases that are important in metabolizing various compounds that regulate behavior, development, insecticidal resistance, and other processes.
RNAi involves silencing the expression of a specific gene by double-stranded RNA (dsRNA) pieces (either small interfering RNA or microRNA each containing about 21-23 nucleotide pairs) attaching to messenger RNA (mRNA) carrying the code from DNA and thus interfering with the production of a specific protein. RNAi is also known as post-transcriptional gene silencing because the silencing is done after the DNA code is transcribed to mRNA. RNAi is a natural phenomenon that helps organisms to defend against infections or regulate gene expression. For example, when there is a viral infection, cells activate RNAi to destroy virus particles. RNAi-based therapies are currently used in the medical field to treat cancer and neurological issues and to regulate oxalic acid in urine or the low-density lipoprotein cholesterol in blood.
RNAi can be used in agriculture for improving yield or quality, imparting abiotic stress tolerance or pest resistance, and incorporating other desirable traits or as biopesticides in crop protection (Bharathi et al., 2023; Chaudhary et al., 2024). Many research studies have been exploring the RNAi potential in agriculture for decades (Fletcher et al., 2020). Modifying plant height in apple (Zhao et al., 2016), rice (Qiao et al., 2007), and tomato (Cheng et al., 202); imparting drought, salt, and heat tolerance in cotton (Abdurakhmonov et al., 2014), abiotic stress tolerance in cereal crops (Dubrovna et al., 2023), and cold tolerance in tomato (Jiao et al., 2024); imparting resistance to blast (Magnaporthe grisea) and leaf blight (Xanthomonas oryzae pv. oryzae) in rice (Jiang et al., 2009), citrus canker (Xanthomonas citri subsp. citri) in citrus (Enrique et al., 2011), late blight (Phytophthora infestans) in potato (Eschen-Lippold et al., 2012), Fusarium head and seedling blight (Fusarium graminearum) in wheat (Cheng et al., 2015), soybean mosaic virus in soybean (Kim et al., 2016); imparting resistance to bollworm (Helicoverpa armigera) in cotton (Mao et al., 2007 and 2011) and resistance to brown planthopper (Nilaparvata lugens) in rice (Zha et al., 2011); and imparting resistance to root-knot nematode (Meloidogyne incognita) in tomato (Dutta et al., 2015) and soybean cyst nematode (Heterodera glycines) in soybean (Guo et al., 2015) are some of the examples of improving crop traits.
The first RNAi crop in the United States is corn (SmartStax® PRO) against the western corn rootworm (Diabrotica virgifera virgifera) containing both Bacillus thuringiensis toxins and RNAi technology (Head et al., 2017). With its ability to resist both below- and above-ground lepidopteran pests, this hybrid is an important IPM tool. This hybrid is also available in Canada for cultivation, and grain and products from the hybrid are approved for consumption in the European Union. RNAi-based crops are not considered genetically modified organisms (GMOs) because they do not contain a foreign gene to express a particular protein like GMOs but use a natural mechanism to silence a particular gene.
In addition to adding desirable traits to crops, RNAi has also been explored or developed for treating plants against pests and diseases. While RNAi crops use the host-induced gene silencing (HIGS) method, RNAi biopesticides use the spray-induced gene silencing (SIGS). SIGS has been explored for controlling Fusarium graminearum in barley (Koch et al., 2016), sucking and/or stem-boring insects in multiple crops (Li et al. 2015; Hunter and Wintermantel, 2021; Jain et al., 2022), hawthorn spider mite (Amphitetranychus viennensis) in fruit trees and woody ornamentals (Yang et al., 2023). The first sprayable formulation of RNAi-based biopesticide is CalanthaTM from GreenLight Biosciences against the Colorado potato beetle (CPB), Leptinotarsa decemlineata (Rodrigues et al., 2021). The active ingredient is a dsRNA molecule known as Ledprona (Leptinotarsa decemlineata-specific recombinant double-stranded interfering Oligonucleotide GS2). It belongs to a new class of insecticides under group 35 as an RNAi-mediated target suppressor. Applied as a foliar spray, Ledprona suppresses the gene that produces proteasome subunit beta type-5 (PSBT5) in CPB and arrests insect feeding within 2-3 days after it is ingested leading to the death of the pest. PSBT5 is an essential protein important in maintaining cellular protein quality by degrading damaged or misfolded proteins or proteins that are no longer needed.
RNAi can also be used to protect honey bees from the Israeli Acute Paralysis Virus (Hunter et al., 2010) and the Varroa mite (Garbian et al., 2012). In field studies, honey bee populations and honey production increased when bees were fed dsRNA for the virus in the presence of virus in the colonies (Hunter et al., 2010). The ectoparasite Varroa mite is a major threat to the honey bee colony health and its management is a significant challenge. When honey bees ingest the mite-specific dsRNA that silences the calcium ion-binding protein known as calmodulin, the dsRNA is transmitted to the Varroa mite feeding on the hemolymph of the bees resulting in mite mortality (Garbian et al., 2012).
As with any new technology, it is important to consider the impact of RNAi on the environment and non-target organisms. Environmental risks and regulatory aspects of RNAi-based products have been reviewed in various reports (Liu et al., 2021; De Schutter et al., 2022; Christiaens et al., 2022). Microbial activity, UV radiation, and other environmental conditions degrade dsRNA and they are generally less stable in the environment, especially under the field conditions where they are used (Bachman et al., 2020). Studies showed that dsRNA degraded within two days in soil and 1-3 days in the aquatic environment (Dubelman et al., 2014; Fishcer et al., 2017). Chen et al. (2023) reported that while an RNAi-based biopesticide was highly effective against the 28-spotted ladybeetle (Henosepilachna vigintioctopunctata), a pest of solanaceous crops, it had no non-target effect on the predatory lady beetle Propylea japonica. Similarly, studies showed that the dsRNA developed for controlling Varroa mite were safe for honey bees (Tan et al., 2016; Vélez et al., 2016) and the monarch butterfly (Danaus plexxippus) whose calmodulin mRNA has a slight match to the Varroa-active dsRNA (Krishnan et al., 2021).
With regards to Ledprona, the US Environmental Protection Agency (EPA) found that it has minimal human and environmental risks due to low application rates, rapid microbial degradation in the environment, and physiological barriers and degradation mechanisms in mammals. EPA also gave Ledprona a “No Effect” determination according to the Endangered Species Act.
Environmental instability is one of the concerns for SIGS but formulation technology can address this problem. Instead of spraying naked dsRNA, formulating it with layered double hydroxide clay nanoparticles known as BioClay significantly extended the stability of dsRNA. Spraying dsRNA in BioClay provided protection against pepper mild mottle virus and cucumber mosaic virus at least for 20 days and dsRNA was detected on the leaves 30 days after application (Mitter et al., 2017). Similarly, spraying BioClay-formulated dsRNA 5 days before exposing to virus-containing green peach aphids (Myzus persicae) offered protection against the bean common mosaic virus in cowpea and benth (Nicotiana benthamiana) (Worrall et al., 2019). In a more recent study, BioClay-formulated dsRNA against gray mold (Botrytis cenerea) increased disease protection from 1 week to 3 weeks on leaves and 5 days to 10 days on fruit (Niño-Sánchez et al., 2022).
Arthropod pests and pathogens are resilient and rapidly evolving organisms and can develop resistance to RANi technology just like they develop to pesticides or transgenic crops. Whether it is HIGS or SIGS, avoiding heavy reliance on one tool and adopting integrated pest management (IPM) and resistance management strategies is crucial even when using RNAi. An IPM strategy that takes advantage of multiple tools will minimize the risk of resistance development while achieving desired pest suppression.
Additional resources:
Videos about RNAi: https://youtu.be/xDg6pu7HWz4 and https://youtu.be/cK-OGB1_ELE
References
Abdurakhmonov, I. Y., Z. T. Buriev, S. Saha, J. N. Jenkins, A. Abdukarimov and A. E. Pepper. 2014. Phytochorme RNAi enhances major fibre quality and agronomic traits of the cotton Gossypium hirsutum L. Nat. Comm. 5: 3062. https://doi.org/10.1038/ncomms4062.
Backman, P., J. Fischer, Z. Song, E. Urbanczyk-Wochniak and G. Watson. 2020. Environmental fate and dissipation of applied dsRNA in soil, aquatic systems, and plants. Front. Plant Sci. 11: 508351. https://doi.org/10.3389/fpls.2020.00021.
Bharathi, J. K., R. Anandan, L. K. Benjamin, S. Muneer, and M.A.S. Prakash. 2023. Recent trends and advances of RNA interference (RNAi) to improve agricultural crops and enhance their resilience to biotic and abiotic stresses. Plant Physiol. Biochem. 194: 600-618.
Chaudhary, D., A. S. Jeena, S. Gaur, R. Raj, S. Mishra, O. P. Gupta, and M. R. Meena. 2024. Advances in RNA interference for plant functional genomics: unveiling traits mechanisms, and future directions. Appl. Biochem. Biotechnol. https://doi.org/10.1007/s12010-023-04850-x.
Chen, S. X. Luo, S. Nanda, C. Yang, Z. Li, Y. Zhang, X. Zhou and H. Pan. 2023. RNAi-based biopesticides against 28-spotted ladybeetle Henosepilachna vigintioctopunctata does not harm the insect predator Propylea japonica. J. Agric. Food Chem. 71: 3373-3384.
Cheng, W., S. Yin, Y. Tu, H. Mei, Y. Wang and Y. Yang. 2020. SICAND1, encoding cullin-associated NEdd8-dissociated protein 1, regulates plant height, flowering time, seed germination, and root architecture in tomato. Plant Mol. Biol. 102: 537-551. https://doi.org/10.1007/s11103-020-00963-7.
Cheng, W., X.-S. Song, H.-P. Li, L.-H. Cao, K. Sun, X.-L. Qiu, Y.-B. Xu, P. Yang, T. Huang, J.-B. Zhang, B. Qu and Y.-C. Liao. 2015. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 13: 1335-1345. https://doi.org/10.1111/pbi.12352.
Christiaens, O., J. Sweet, T. Dzhambazova, I. Urru, G. Smagghe, K. Kostov and S. Arpaia. 2022. Implementation of RNAi-based arthropod pest control: environmental risks, potential for resistance and regulatory considerations. J. Pest Sci. 95: 1-15. https://doi.org/10.1007/s10340-021-01439-3.
De Schutter, K., C.N.T. Taning, L. Van Daele, E.J.M. Van Damme, P. Dubruel and G. Smagghe. 2022. RNAi-based biocontrol products: market status, regulatory aspects, and risk assessment. Front. Insect Sci. 1: 818037. https://doi.org/10.3389/finsc.2021.818037.
Dubelman, S., J. Fischer, F. Zapata, K. Huizinga, C. Jiang, J. Uffman, S. Levine and D. Carson. 2014. Environmental fate of double-stranded RNA in agricultural soils. PLoS One. https://doi.org/10.1371/journal.pone.0093155.
Dubrovna, O. V., S. I Mykhalska, and A. G. Komisarenko. 2023. Use of RNA interference technology for improving economically valuable traits of cereal crops. Cytology and Genetics 57: 587-610.
Dutta, T. K., P. K. Papolu, P. Banakar, D. Choudhary, A. Sirohi and U. Rao. 2015. Tomato transgenic plants expressing hairpin construct of a nematode protease gene conferred enhanced resistance to root-knot nematodes. Front. Microbiol. 6: 260. https://doi.org/10.3389/fmicb.2015.00260.
Enrique, R., F. Siciliano, M. A. Favaro, N. Gerhardt, R. Roeschlin, L. Rigano and M. R. Marano. 2011. Novel demonstration of RNAi in citrus reveals importance of citrus callose synthase in defence against Xanthomonas citri subsp. citri. Plant Biotehnol. J. 9: 394-407. https://doi.org/10.1111/j.1467-7652.2010.00555.x.
Eschen-Lippold, L., R. Ladgraf, U. Smolka, S. Schulze, M. Heilmann, I. Heilmann, G. Hause and S> ROsahl. 2012. Activation of defense against Phytophthora infestans in potato by down-regulation of syntaxin gene expression. The Ne Phytologist 193: 985-996. https://doi.org/10.1111/j.1469-8137.2011.04024.x.
Fischer, J. R., F. Zapata, S. Dubelman, G. M. Mueller, J. P. Uffman, C. Jiang, P. D. Jensen and S. L. Levine. 2017. Aquatic fate of a double-stranded RNA in a sediment-water system following an over-water application. Environ. Toxicol. Chem. 36: 727-734. https://doi.org/10.1002/etc.3585.
Fletcher, S. J., P. T. Reeves, B. T. Hoang, and N. Mitter. 2020. A perspective on RNAi-based biopesticides. Frontiers in Plant Science 11: 51. https://doi.org/10.3389/fpls.2020.00051.
Garbian, Y., E. Maori, H. Kalev, S. Shafir and I. Sela. 2012. Bidirectional transfer of RNAi between honey bee and Varroa destructor: Varroa gene silencing reduces Varroa population. PLoS Pathogens 8: e1003035. https://doi.org/10.1371/journal.ppat.1003035.
Guo, X., D. Chronis, C. M. De La Torre, J. Smeda, X. Wang and M. G. Mitchum. 2015. Enhanced resistance to sybean cyst nematode Heterodera glycines in transgenic soybean by silencing putative CLE receptors. Plant Biotechnol. J. 13: 801-810. https://doi.org/10.1111/pbi.12313.
Head, G. P., M. W. Carroll, S. P. Evans, D. M. Rule, A. R. Willse, T. L. Clark, N. P. Storer, R. D. Flannagan, L. W. Samuel and L. J. Meinke. 2017. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: efficacy and resistance management. Pest Manag. Sci. 73: 1883-1899. https://doi.org/10.1002/ps.4554.
Hunter, W., J. Ellis, D. vanEngelsdorp, J. Hayes, D. Westervelt, E. Glick, M. Williams, I. Sela, E. Maori, J. Pettis, D. Cox-Foster and N. Paldi. 2010. Large-scale field application of RNAi technology reducing Israili Acute Paralysis Virus disease in honey bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathogens 6: e1001160. https://doi.org/10.1371/journal.ppat.1001160.
Hunter, W. B. and W. M. Wintermantel. 2021. Optimizing efficient RNAi-mediated control of hemipteran pests (psyllids, leafhoppers, whitefly): modified pyrimidines in drRNA triggers. Plants 10: 1782. https://doi.org/10.3390/plants10091782.
Jain, R. G., S. J. Fletcher, N. Manzie, K. E. Robinson, P. Li, E. Lu, C. A. Brosnan, Z. P. Xu and N. Mitter. 2022. Foliar application of clay-delivered RNA interference for whitefly control. Nature Plants 8: 535-548.
Jiang, C.-J., M. Shimono, S. Maeda, H. Inoue, M. Mori, M. Hasegawa, S. Sugano and H. Takatsuji. 2009. Suppression of the rice fatty-acid desaturase gene OsSSI2 enhances resistance to blast and leaf blight diseases in rice. Mol. Reprod. Dev. 22: 820-829. https://doi.org/10.1094/MPMI-22-7-0820.
Jiao, C., J. Sun. and Y. Wei. 2024. SlWRKY31 enhances chilling tolerance by interacting with SlSIZ1 in tomato fruit. Postharvest Biol. Technol. 207: 112631. https://doi.org/10.1016/j.postharvbio.2023.112631.
Kim, H. J., M. J. Kim, J. H. Pak, H. H. Im, D. H. Lee, K. H. Ki, and Y. S. Chung. 2016. RNAi-mediated soybean mosaic virus (SMV) resistance of a Korena soybean cultivar. Plant Biotechnol. Reports 10: 257-267. https://doi.org/10.1007/s11816-016-0402-y.
Koch, A., D. Biedenkopf, A. Furch, L. Weber, O. Rossbach, E. Abdellatef, L. Linicus, J. Johannsmeier, L. Jelonek, A. Goesmann, V. Cardoza, J. McMillan, T. Mentzel and K.-H. Kogel. 2016. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathogens 12: e1005901. https://doi.org/10.1371/journal.ppat.1005901.
Krishnan, N., M. J. Hall, R. L. Hellmich, J. R. Coats and S. P. Bradbury. 2021. Evaluating toxicity of Varroa mite (Varroa destructor)-active dsRNA to monarch butterfly (Danaus Plexippus) larvae. PLoS One 16: e0251884. https://doi.org/10.1371/journal.pone.0251884.
Li, H. R. Guan, H. Guo and X. Miao. 2015. New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant, Cell & Environment 38: 2277-2285. https://doi.org/10.1111/pce.12546.
Liu, S., S. Geng, A. Li, Y. Mao and L. Mao. 2021. RNAi technology for plant protection and its application in wheat. aBIOTECH 2: 365-374. https://doi.org/10.1007/s42994-021-00036-3.
Mao, Y. B., W. J. Cai, J. W. Wang, G. J. Hong, X. Y. Tao, L. J. Wang and X. Y. Chen. 2007. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotehnol. 25: 1307-1313. https://doi.org/10.1038/nbt1352.
Mao, Y. B., X. Y. Tao, X. Y. Xue, L. J. Wang and X. Y. Chen. 2011. Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Trans. Res. 20: 665-673. https://doi.org/10.1007/s11248-010-9450-1.
Mitter, N., E. A. Worrall, K. E. Robinson, P. Li, R. G. Jain, C. Taochy, S. J. Fletcher, B. J. Carroll, G. Q. Lu and Z. P. Xu. 2017. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 3: 16207. https://doi.org/10.1038/nplants.2016.207.
Niño-Sánchez, J., P. T. Sambasivam, A. Sawyer, R. Hamby, A. Chen, E. Czislowski, P. Li, N. Manzie, D. M. Gardiner, R. Ford, Z. P. Xu, N. Mitter and H. Jin. BioClayTM prolongs RNA interference-mediated crop protection against Botrytis cinerea. J. Integrative Pl. Biol. 64: 2187-2198. https://doi.org/10.1111/jipb.13353.
Qiao, F., Q. Yang, C. L. Wang, Y. L. Fan, X. F. Wu, and K. J. Zhao. 2007. Modification of plant
height via RNAi suppression of OsGA20ox2 gene in rice. Euphytica 158: 35–45.
https://doi.org/10.1007./s10681-007-9422-6.
Rodrigues, T., K. Sridharan, B. Manley, D. Cunningham and K. Narva. 2021. Development of dsRNA as a sustainable bioinsecticide: from laboratory to field. In: Rauzan BM and Lorsbach BA, editors. Crop protection Products for Sustainable Agriculture, ACS Symposium Series. 1390. Washington, DC: ACS Publications, p. 65–82.
Tan J., S. L. Levine, P. M. Bachman, P. D. Jensen, G. M. Mueller, J. P. Uffman, C. Meng, Z. Song, K. B. Richards and M. H. Beevers. 2016. No Impact of DvSnf7 RNA on Honey Bee (Apis Mellifera L.) Adults and Larvae in Dietary Feeding Tests. Environ. Toxicol. Chem. 35: 287–294. https://doi.org/10.1002/etc.3075.
Vélez, A. M., J. Jurzenski, N. Matz, X. Zhou, H. Wang, M. Ellis and B. D. Siegfried. 2016. Developing an in Vivo Toxicity Assay for RNAi Risk Assessment in Honey Bees, Apis Mellifera L. Chemosphere 144: 1083–1090. https://doi.org/10.1016/j.chemosphere.2015.09.068.
Worrall, E. A., A. Bravo-Cazar, A. T. Nilon, S. J. Fletcher, K. E. Robinson, J. P. Carr and N. Mitter. 2019. Exogenous application of RNAi-induced double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Front. Plant Sci. 10: 265. https://doi.org/10.3389/fpls.2019.00265.
Yang, J., Y. Zhang, J. Zhao, Y. Gao, Z. Liu, P. Zhang, R. Fan, S. Xing and X. Zhou. 2023. Target gene selection for RNAi-based biopesticides against the hawthorn spider mite, Amphitetranychus viennensis (Acari: Tetranychidae). Pest Manag. Sci. 79: 2482-2492.
Zha, W., X. Peng, R. Chen, B. Du, L. Zhu, and G. He. 2011. Knockdown of midgut genes by dsRNA-transgenic plant-mediated RNA interference in the hemipteran insect Nilaparvata lugens. PLoS One 6: e20504. https://doi.org/10.1371/journal.pone.0020504.
Zhao, K., F. Zhang, Y. Yang, Y. Ma, Y. Liu, H. Li, and Z. Zhang. 2016. Modification of plant height via RNAi suppression of MdGA20-ox gene expression in apple. J. Am. Soc. Hort. Sci. 141: 242-248. https://doi.org/10.21273/JASHS.141.3.242.
- Author: Surendra K. Dara
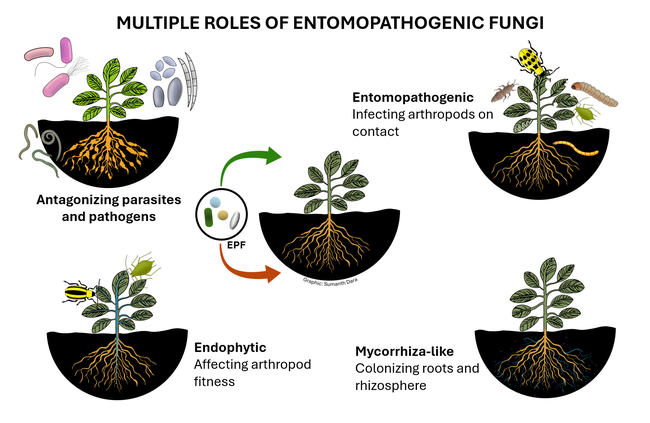
Entomopathogenic fungi (EPF) are those that infect various arthropods such as ticks, mites, and insects. There are two major groups of EPF that play an important role in pest suppression. Members of the order Entomophthorales are more host-specific and examples include Entomophaga maimaiga in spongy moth, Entomophthora muscae and Strongwellsea spp. in flies, Conidiobolus obscurus, Entomophthora planchoniana, Neozygites fresenii and Pandora neoaphidis in aphids, and Neozygites floridana in mites. These naturally occurring EPF are fastidious and cannot be mass produced on a commercial scale, but cause epizootics when host populations are high and environmental conditions are favorable resulting in significant pest suppression. On the other hand, members of the order Hypocreales are more generalistic pathogens and can be infective to a variety of arthropods. Beauveria bassiana, Cordyceps fumosorosea, Hirsutella thompsonii, and Metarhizium brunneum are some examples of hypocrealeans. These can be grown on artificial media on a commercial scale and several biopesticide formulations based on various isolates of these fungi are available in the US and elsewhere. Both entomophthoralean and hypocrealean fungi have the same mode of infection. When fungal spores come in contact with their host, they germinate and enter the host body through mechanical pressure and enzymatic degradation of the cuticle. They multiply inside the host, invade the tissues, and finally emerge from the cuticle to produce spores that continue the infection process.
With growing emphasis on sustainable crop production with safer pesticides, the market for biopesticides including EPF-based ones has been increasing. Newer EPF isolates and modern technology contributed to the development of improved formulations. EPF-based products can be used for soil-inhabiting pests or their life stages like root aphids, pupae of thrips, and wireworms to foliar feeders or above-ground pests including the members of Coleoptera, Diptera, Hemiptera, Orthoptera, Thysanoptera, and others. Considering their potential against a variety of pests on multiple crops, EPF-based pesticides should be an important part of integrated pest management (IPM) programs. However, there is a significant knowledge gap in effectively using EPF in IPM and fully exploring their potential in sustainable crop production.
Since EPF spores need to come in contact with the host, using them against the right pest or life stage is very important to obtain desired results. Sometimes, using EPF in combination or rotation with botanical or synthetic pesticides is more effective than using them alone against a particular pest (Dara 2013; 2015; 2016). As EPF formulations contain live fungi, label instructions should be followed for proper storage, transportation, tank-mixing, and application to maintain their efficacy. Compatibility can vary according to the EPF and its formulation, but studies showed that some isolates of Beauveria bassiana and Metarhizium anisopliae are compatible with several fungicides (Dara, et al., 2014; Roberti et al., 2017; Khun et al., 2021).
In addition to controlling arthropod pests, EPF being soilborne fungi also have a direct relationship with plants and other microbes. EPF colonize plant tissues and grow inside the plants in a phenomenon known as endophytism. Endophytic EPF grow as hyphae and do not produce spores. Although they cannot cause infection to pests feeding on those plants, they indirectly affect pests by reducing their fitness and survival by activating induced systemic resistance. When EPF are applied to soil, they form a mycorrhiza-like relationship with plant roots and help plants withstand biotic stresses and improve nutrient uptake. EPF can also antagonize plant pathogens through competitive displacement and antimicrobial activity. Thus soil and foliar application of EPF-based pesticides result in additional benefits in improving crop growth and health in addition to controlling pests through infection.
Several studies explored the non-entomopathogenic roles of EPF (Dara, 2019a). Soil application of B. bassiana had a positive impact on the survival, growth, and health of cabbage plants growing under water stress (Dara et al., 2017). Metarhizium brunneum also had a similar impact on plant growth in this study. Root and rhizosphere colonization by Metarhizium spp.improved shoot length and root weight in industrial hemp (Hu et al., 2023) and root colonization of Metarhizium robertsii alleviated hemp from salt and drought stress. Metarhizium spp. and B. bassiana transferred nitrogen from dead insects to the plant they colonized (Behie et al., 2012; Behie and Bidochka, 2014). These studies show the role of EPF in soil nitrogen cycle and how plants benefit from the endophytic relationship of EPF. Additionally, recent reports showed that endophytic B. bassiana induced the biosynthesis of flavonoids in oilseed rape (Muola et al., 2023) and flavonol content in licorice plants (Etsassala et al., 2023).
Seed treatment with B. bassiana increased plant height, stem diameter, number of leaves, shoots and apical buds, biomass, and total chlorophyll content in cotton and reduced cotton aphid (Aphis gossypii) populations (Mantzoukas et al., 2023). Similarly, endophytic B. bassiana significantly reduced the reproductive rate and populations of the Russian wheat aphid (Diuraphis noxia) in South African wheat (Motholo et al., 2023). In corn, endophytic B. bassiana and M. anisopliae negatively impacted the survival, development, and reproduction of the fall armyworm (Spodoptera frugiperda) (Altaf et al., 2023).
Soil application of B. bassiana, Cordyceps fumosorosea, and Metarhizium brunneum antagonized Fusarium oxysporum f.sp. vasinfectum in cotton as effectively as some biofungicides (Dara et al., 2020). Beauveria bassiana treatment at a higher rate provided significantly better protection than all other treatments in this study. Both B. bassiana and C. fumosorosea inhibited the growth of F. oxysporum in vitro (Yanagawa et al., 2021). In corn, endophytic M. robertsii promoted plant growth and reduced southern corn leaf blight caused by Cochliobolus heterostrophus (Imtiaz et al., 2023). Induced systemic resistance is thought to be responsible for this protection. Similarly, B. bassiana applied as seed treatment, seedling root dip, and foliar spray reduced the incidence of rice sheath blight caused by Rhizoctonia solani by 69% and its severity by 60% under field conditions (Deb et al., 2023). Beauveria bassiana also resulted in 71% of mycelial inhibition in R. solani through the production of cell wall degrading enzymes, release of secondary metabolites, and mycoparasitism.
Multiple recent studies showed that EPF also have a negative impact on plant-parasitic nematodes. Beauveria bassiana and C. fumosorosea reduced the survival ofthe root-knot nematode, Meloidogyne incognita, in vitro (Yanagawa et al., 2021). Similar to the nematophagous fungus Purpureocillium lilacinum, both B. bassiana and M. anisopliae were effective in reducing galls caused by M. incognita in tomato and cucumber (Karabörklü et al., 2022). Metarhizium anisopliae was as effective as P. lilacinum with 75% reduction in gall formation and 85% control of second instar juveniles in tomato. Beauveria bassiana and M. anisopliae also resulted in about 85% control of second instar juveniles in cucumber. In another study, soil application of B. bassiana significantly reduced nematode infestation in tomato roots and B. bassiana treatment caused 60% mortality in nematodes in a lab assay (Kim et al., 2023). Volatile organic compounds, 1-octen-3-ol and 3-octanone from M. brunneum attracted and killed another plant-parasitic nematode, Meloidogyne hapla, in lab assays (Khoja et al., 2021).
As many of these recent studies indicated, the non-entomopathogenic roles of EPF is a new area of applied research interest with tremendous practical benefits. In addition to direct pest control through infection, EPF as endophytes offer multiple benefits in suppressing pest populations by affecting their fitness, antagonizing plant pathogens and plant-parasitic nematodes, imparting drought and salt tolerance in plants, improving nutrient uptake, and promoting overall growth and health of plants. Using EPF-based biopesticides comes under the microbial control of IPM (Dara, 2019b) and will contribute to insecticide resistance management. Additionally, the non-target benefits of EPF will help growers optimize the use of other inputs and related costs. EPF can be very important in sustainable crop production and a thorough understanding of their biology, interactions with pests, plants, pathogens, and other biotic and abiotic factors, and effective use strategies will help achieve their full potential.
Note: This article was initially published in the December 2023 issue of CAPCA Adviser magazine.
References
Altaf, N., M. I. Ullah, M. Afzal, M. Arshad, S. Ali, M. Rizwan, L. A. Al-Shuraym, S. S. Alhelaify, and S. Sayed. 2023. Endophytic colonization by Beauveria bassiana and Metarhizium anisopliae in maize plants affects the fitness of Spodoptera frugiperda (Lepidoptera: Noctuidae). Microorganisms 11: 1067.
Behie, S. W. and M. J. Bidochka. 2014. Ubiquity of insect-derived nitrogen transfer to plants by endophytic insect-pathogenic fungi: an additional branch of the soil nitrogen cycle. Appl. Environ. Microbiol. 80: 1553-1560.
Behie, S. W., P. M. Zelisko, and M. J. Bidochka. 2012. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 336: 1576-1577.
Dara, S. 2013. Microbial control as an important component of strawberry IPM. CAPCA Adviser, 16 (1): 29-32.
Dara, S. K. 2015. Root aphids and their management in organic celery. CAPCA Adviser 18 (5): 65-70.
Dara, S. K. 2016. IPM solutions for insect pests in California strawberries: efficacy of botanical, chemical, mechanical, and microbial options. CAPCA Adviser 19 (2): 40-46.
Dara, S. K. 2019a. Non-entomopathogenic roles of entomopathogenic fungi in promoting plant health and growth. Insects 10: 277.
Dara, S. K. 2019b. The new integrated pest management paradigm for the modern age. JIPM 10: 12.
Dara, S. K., S. S. Dara, and S.S.R. Dara. 2020. Managing Fusarium oxysporum f. sp. vasinfectum Race 4 with beneficial microorganisms including entomopathogenic fungi. Acta Horticulturae 1270: 111-116.
Dara, S. K., S.S.R. Dara, and S. S. Dara. 2017. Impact of entomopathogenic fungi on the growth, development, and health of cabbage growing under water stress. Am. J. Plant Sci. 8: 1224-1233.
Dara, S.S.R., S.S. Dara, A. Sahoo, H. Bellam, and S. K. Dara. 2014. Can entomopathogenic fungus Beauveria bassiana be used for pest management when fungicides are used for disease management? UCANR eJournal of Entomology and Biologicals October 23, 2014.
Deb, L., P. Dutta, M. K. Mandal and S. B. Singh. 2023. Antimicrobial traits of Beauveria bassiana against Rhizoctonia solani, the causal agent of sheath blight of rice under filed conditions. Plant Disease PDIS-04. https://doi.org/10.1094/PDIS-04-22-0806-RE.
Etsassala, N. G. E. R., N. Macuphe, I. Rhoda, F. Rautenbach and F. Nchu. 2023. An endophytic Beauveria bassiana (Hypocreales) strain enhances the flavonol contents of Helichrysum petiolare. In Sustainable Uses and Prospects of Medicinal Plants, eds. L. Kambizi and C Bvenura, CRC Press. pp 367-377.
Hu, S. and M. J. Bidochka. 2023. Colonization of hemp by Metarhizium and alleviation of salt and drought stress. 55th Annual meetings of the Society for Invertebrate Pathology, July 30-August 3, 2023, College Park, MD, pp. 56-57.
Hu, S., M. S. Mojahid, M. J. Bidochka. 2023. Root colonization of industrial hemp (Cannabis sativa L.) by the endophytic fungi Metarhizium and Pochonia improves growth. Industrial Crops and Products 198: 116716.
Imtiaz, A. M. M. Jiménez-Gasco, and M. E. Barbercheck. 2023. Endophytic Metarhizium robertsii suppresses the phytopathogen, Cochliobolus heterostrophus and modulates maize defenses. 55th Annual meetings of the Society for Invertebrate Pathology, July 30-August 3, 2023, College Park, MD, pp. 66-67.
Karabörklü, S., V. Aydinli and O. Dura. 2022. The potential of Beauveria bassiana and Metarhizium anisopliae in controlling the root-knot nematode Meloidogyne incognita in tomato and cucumber. J. Asia-Pacific Entomol. 25: 101846.
Khoja, S., K. M. Eltayef, I. Baxter, A. Myrta, J. C. Bull and T. Butt. 2021. Volatiles of the entomopathogenic fungus, Metarhizium brunneum, attract and kill plant parasitic nematodes. Biol. Con. 152: 104472.
Khun, K. K., G. J. Ash, M. M. Stevens, R. K. Huwer, B. A. Wilson. 2021. Compatibility of Metarhizium anisopliae and Beauveria bassiana with insecticides and fungicides used in macadamia production in Australia. Pest Manag. Sci. 77: 709-718.
Kim, K. J., S. E. Park, Y. Im, H. Yang and J. S. Kim. 2023. Drenching of Beauveria bassiana JEF-503 reduces the root knot nematode populations in soil. 55th Annual meetings of the Society for Invertebrate Pathology, July 30-August 3, 2023, College Park, MD, pp. 68.
Mantzoukas, S., V. Papantzikos, S. Katsogiannou, A. Papanikou, C. Koukidis, D. Servis, P. Eliopoulos, and G. Patakioutas. 2023. Biostimulant and bioinsecticidal effect of coating cotton seeds with endophytic Beauveria bassiana in semi-field conditions. Microorganisms 11: 2050.
Motholo, L. F., M. Booyse, J. L. Hatting, T. J. Tsilo, M. Lekhooa, and O. Thekisoe. 2023. Endophytic effect of the South African Beuaveria bassiana strain PPRI 7598 on the population growth and development of the Russian wheat aphid, Diuraphis noxia. Agriculture 13: 1060.
Moula, A., T. Birge, M. Helander, S. Mathew, V. Harazinova, K. Saikkonen, and B. Fuchs. 2023. Endophytic Beauveria bassiana induces biosynthesis of flavonoids in oilseed rape following both seed inoculation and natural colonization. Pest Manag. Sci. DOI 10.1002/ps.7672
Roberti, R. H. RIghini, A. Masetti, and S. Maini. 2017. Compatibility of Beauveria bassiana with fungicides in vitro and on zucchini plants infested with Trialeurodes vaporariorum. Biol. Con. 113: 39-44.
Yanagawa, A., N.P.R.A. Krishanti, A. Sugiyama, E. Chrysanti, S. K. Ragamustari, M. Kubo, C. Furumizu, S. Sawa, S. K. Dara, and M. Kobayashi. 2022. Control of Fusarium and nematodes by entomopathogenic fungi for organic production of Zingiber officinale. J. Natural Medicines, 76: 291-297.
The western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) is one of the major pests of lettuce in California. It has a wide host range including several vegetable, ornamental, and other cultivated or wild plants. Native to North America, the western flower thrips is also known as alfalfa thrips, California thrips, and maize thrips among others. This article provides a general overview of the pest, its biology, damage, and management.
Biology:
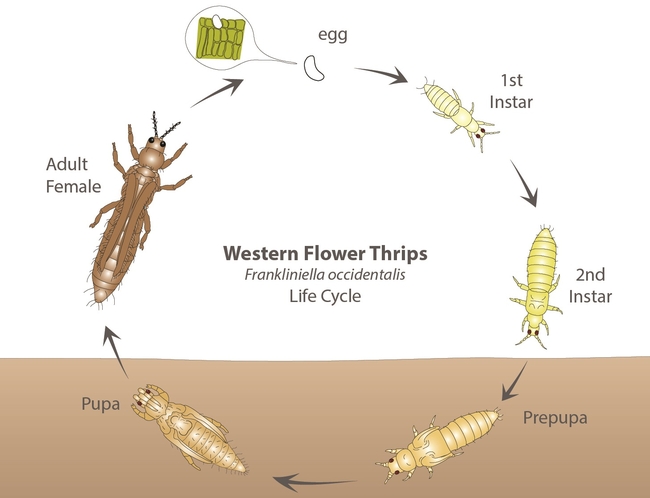
Eggs are small, oval, and inserted into plant tissue. Nymphs are slender and have four instars. The first two - larva I and II – feed on plant tissues while the latter two - prepupa and pupa – are non-feeding stages that are often found in the soil. Larvae are wingless and white initially and turn yellow or orange once they start feeding. Adults are small (< 2 mm), slender, and have two pairs of long, narrow wings with a fringe of hairs. The western flower thrips can occur in different color morphs such as yellow or orange, brown, and black.
Damage:
The western flower thrips prefers flowers, but also feeds on developing buds, fruits, and foliage. Larvae and adults rupture the leaf surface with their rasping mouthparts and feed on plant juices. Feeding damage results in silvery appearance of the leaf surface, which later turns brown. The presence of dark fecal specs indicates thrips occurrence. In lettuce, the western flower thrips transmits Tomato spotted wilt virus and is the sole vector of Impatiens necrotic spot virus. Only the larval stages acquire these tospoviruses and the adults transmit the viruses to other plants as they spread in the field.
Management:
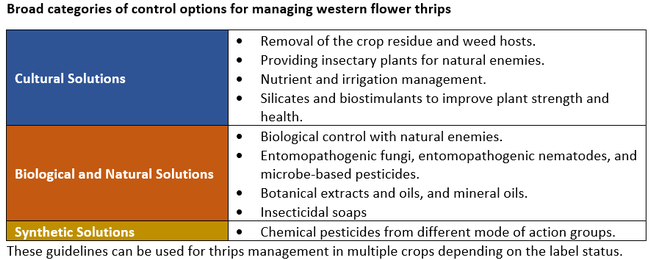
Integrated pest management approach is critical for successful pest management. It involves regular monitoring, exploring the potential of multiple options including cultural and biological solutions, and proper timing and application of various strategies among others. The western flower thrips is one of the pests where insecticide resistance is a common problem. To reduce the risk of resistance development, it is necessary to explore the potential of multiple control options and rotate insecticides with different modes of action. This is essential to suppress pest populations to desired levels and also to maintain control efficacy of existing pesticides.
Cultural control – Remove weed and other hosts that harbor thrips or viruses. Sprinkler irrigation can help reduce thrips populations. Plow down lettuce crop residue to destroy surviving stages. In general, maintaining good plant health with optimal nutrition and irrigation practices helps plants withstand pest damage. Silicate products can improve the structural strength of plant tissues and reduce pest damage and/or populations. Several biostimulants or biological soil amendments can also help activate plant's natural defenses against pest infestations. Consider using them to improve overall plant health and yields, and to protect plants from biotic and abiotic stresses.
Biological control – Predators such lacewings (Chrysopa spp. and Chrysoperla spp.), minute pirate bugs (Orius spp. and Anthocoris spp.), predatory mites (Amblyseius swirski, Ablyseius andersoni, Neoseiulus cucumeris and Stratiolaelaps scimitus), and rove beetles (Dalotia coriaria) attack thrips. Conserve natural enemies with insectary plants and applying safer pesticides, and augment natural populations by releasing commercially reared species.
Microbial control – Entomopathogenic fungi such as Beauveria bassiana and Cordyceps (Isaria) fumosorosea, products based on bacteria such as Burkholderia rinojensis and Chromobacterium subtsugae, and entomopathogenic nematodes such as Heterorhabditis spp. and Steinernema feltiae can be used against one or more life stages. Entomopathogenic nematodes are more effective against pupae in soil because they actively search for and infect their hosts. Entomopathogenic fungi can be used against all life stages.
Botanical control – Azadirachtin alone or in combination with entomopathogenic fungi or insecticides can also be used against multiple life stages. Azadirachtin is an insecticide, antifeedant, and a growth regulator. Similarly, pyrethrins derived from chrysanthemum flowers can be used alone or with other biological or synthetic insecticides. Pyrethrins are nerve poisons. Other botanical insecticides that contain soybean oil, rosemary oil, thymol, and neem oil (which also has a low concentration of azadirachtin) also provide control against thrips through insecticidal, repellency, and antifeedant activities.
Other control options – Insecticidal soaps and mineral oils can be used against different life stages of thrips. Spinosad, a popular insecticide of microbial origin and a mixture of two chemicals spinosyn A and spinosyn D, is very effective against thrips. However, overuse of spinosad can lead to resistance issues in thrips and other insects.
Chemical control – There are several synthetic insecticides that are effective against thrips. It is important to rotate chemicals among different mode of action groups to reduce the risk of insecticide resistance. The following are some synthetic active ingredients and their mode of actions groups in parenthesis that can be used for thrips control: methomyl (1A), bifenthrin (3A), lambda-cyhalothrin (3A), zeta-cypermethrin (3A), clothianidin (4A), spinetoram (5), and cyantraniliprole (28).
Depending on the level of control needed, combinations of products from different categories can improve control efficacy. For example, a combination of entomopathogenic fungi and nematodes can be applied to the soil for controlling prepupae and pupae. While the soil-dwelling predatory mite S. scimitus and the rove beetle, D. coriaria, can be used against pupal stages, other natural enemies can be used against nymphs and adults. A combination of entomopathogenic fungi and azadirachtin can be applied both to the soil or foliage for controlling different life stages. Similarly, various biological and synthetic insecticides can be applied in combination or rotation to obtain desired control.
The categories presented above are based on the source or nature of the active ingredients and do not indicate their organic or conventional label status. Please check the product labels for their appropriateness for managing thrips in lettuce, for use in organic farms, and guidelines for storage, handling, and field use. Entomopathogenic nematodes, fungi, and other biologicals are compatible with several synthetic agricultural inputs, but verify the label guidelines for specific instructions.
Additional resources:
Dara, S. K. 2019. The new integrated pest management paradigm for the modern age. JIPM 10: 1-9. https://doi.org/10.1093/jipm/pmz010
Dara, S. K. 2021. Biopesticides: categories and use strategies for IPM and IRM. UC ANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=46134
Natwick, E. T., S. V. Joseph, and S. K. Dara. 2017. UC IPM pest management guidelines: lettuce. UC ANR Publication 3450. https://www2.ipm.ucanr.edu/agriculture/lettuce/Western-flower-thrips/
Riley, D. G., S. V. Joseph, R. Srinivasan, and S. Diffie. 2011. Thrips vectors of tospoviruses. JIPM 2: I1-I10. https://doi.org/10.1603/IPM10020
- Author: Surendra K. Dara
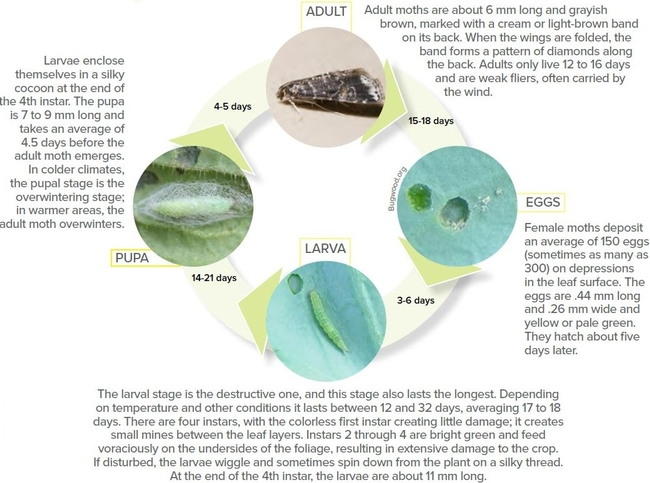
The diamondback moth (DBM), Plutella xylostella, is a small plutellid moth of European origin that has been in North America for nearly two centuries. It is currently present in many parts of the world feeding exclusively on cruciferous hosts such as broccoli, cabbage, and cauliflower. DBM has multiple generations per year and can cause significant yield losses when populations are not controlled. Increasing temperatures that shorten pest life cycle, changing climatic patterns and milder winters in many areas, the ability of adult DBM to disperse, and the presence of cultivated and wild cruciferous crops year-round are worsening the pest problem and require continuous application of pesticides and other control options. Insecticide resistance is also a common problem in DBM where very high levels of resistance to some commonly used pesticides in field populations were reported. Although DBM infestations are common in cruciferous vegetable production, many parts of California and Arizona have seen a significant increase in DBM populations in the past few months. Year-round production of cruciferous vegetables supports DBM populations with as many as 12 generations per year and requires regular application of pesticides. Frequent pesticide applications can lead to insecticide resistance, ineffective pest suppression, and higher yield losses. A good integrated pest management (IPM) strategy is critical to address a pest like DBM.
Biology: A female moth deposits an average of 150 eggs over about 10 days (Capinera, 2018). Eggs are deposited in small batches in depressions on leaf surfaces. Small, green larvae actively feed on the foliage, first instars in mines and the remaining three on the surface. Pupation usually occurs on the lower side of the leaf surface in a loosely spun cocoon. Adult moths are slender, greyish brown with conspicuous antennae. The light-colored diamond pattern on the wings when the moth is resting gives the name diamondback moth.
Damage: Larval feeding on foliage and growing parts of young plants causes skeletonization of leaves. Larvae can also bore into the heads and flower buds resulting in the failure of head formation and stunting of plant growth. Uncontrolled populations cause significant yield losses.
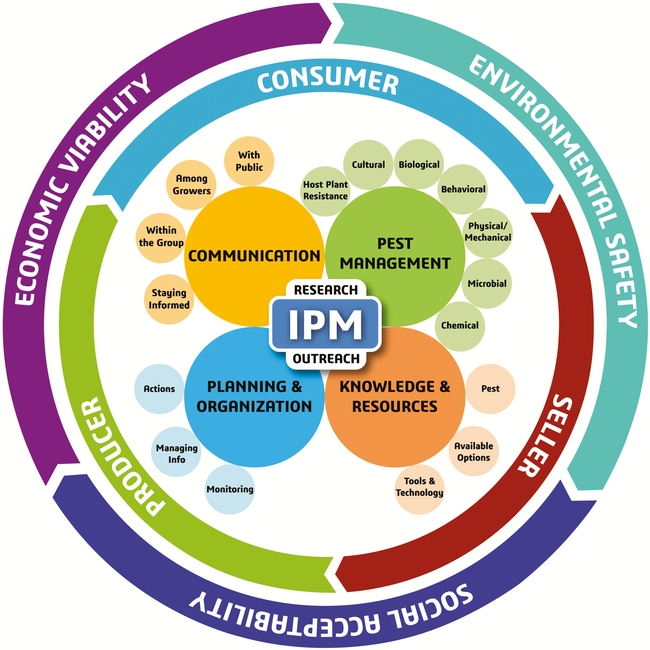
A sound IPM strategy involves regular monitoring of pest infestations, a good understanding of the pest life cycle, and using multiple tactics that target one or more life stages (Dara, 2019). The following recommendations are developed based on the new IPM model and its different components.
A. Pest Management: Some pests can be effectively controlled by one or two tactics, but a difficult pest like DBM with its increasing threat needs a variety of tactics to achieve maximum control.
i) Host plant resistance: Planting cultivars that tolerate or resist DBM damage is the first line of defense. For example, cabbage cultivars with glassy leaves (Dickson et al., 1990) and a specific glucosinolate profile (Robin et al., 2017) are resistant to larval damage. On glassy leaf surfaces, larvae spend less time feeding and more time searching for a suitable spot to feed. The presence or higher levels of glucobrassicin, glucoiberin, and glucoiberverin and the absence or lower levels of 4-hydroxyglucobrassicin, glucoerucin, glucoraphanin, and progoitrin showed resistance to larval feeding in cabbage (Robin et al., 2017).
ii) Cultural control: Maintaining a brassica-free period or rotating with non-brassica crops will help break the pest cycle. Removal of weedy hosts can also reduce the source of infestation, but DBM adults can disperse in search of their hosts. Good agronomic practices can ensure optimal plant health and compensate for potential yield losses when infestations are low. Certain biostimulants can induce systemic resistance or strengthen plant tissues and further contribute to the plant health under pest attack.
iii) Biological control: Various species of natural enemies contribute to the control of DBM (Sarfraz et al., 2007). The egg parasitoid Trichogramma pretiosum and the larval parasitoids Cotesia plutellae, Diadegma insulare, Diadromus subtilicornis, and Microplitis plutellae, predatory ground beetles, hemipterans, syrphid fly larvae, and spiders are some of the natural enemies of DBM. Depending on the availability, parasitoids of other Cotesia spp. and Oomyzus spp. can also be used. Conserving these natural enemies by providing strips of insectary plants in the field along with releasing commercially available natural enemies will provide the necessary biological control of DBM.
iv) Behavioral control: Mating disruption with sex pheromone is the most effective behavioral control tactic for DBM. Using pheromones confuses the male moth in finding its female mate, reduces mating, and thus the next generation individuals. A recent study in a commercial Brussels sprouts field demonstrated the potential of mating disruption with a sprayable pheromone (Dara, 2020). Studies conducted in different countries explored the potential of various antifeedants against DBM larvae and when commercially available, such materials can contribute to DBM IPM. A triterpenoid saponin from the crucifer Barbarea vulgaris in Japan (Shinoda et al., 2002), momordicine I and II from the cucurbit Momordica charantia in China (Ling et al., 2008), and the extracts of Acalypha fruticosa (family Euphorbiaceae) in India (Lingathurai et al., 2011) are some examples of the antifeedant materials investigated against DBM.
v) Physical control: Depending on the field size, crop stage, and affordability, row covers can be used to exclude DBM.
vi) Microbial control: DBM is susceptible to naturally occurring bacterial, fungal, and viral pathogens, but biopesticides based on the bacterium Bacillus thuringiensis and the bacterial toxin spinosad are the most common microbial control options for DBM in the United States. Baculovirus-based products are available for DBM control in other countries.
vii) Chemical control: Application of chemical pesticides of natural and synthetic origin is the most commonly used tactic for DBM control. Azadirachtin, pyrethrins, and synthetic pesticides from different mode of action groups can be used against DBM. Studies conducted in Ethiopia (Begna and Damtew, 2015), India (Devi and Tayde, 2017), and Thailand (Kumrungsee et al., 2014) explored the potential of various botanical extracts against DBM with varying levels of efficacy. Vegetable oils, mineral oils, neem oil, and others can also be used as both ovicides and larvicides.
B. Knowledge and Resources: The most important aspect of IPM is to develop a good understanding of the pest life cycle, seasonal trends, host preference, feeding behavior, response to environmental conditions, and biotic and abiotic stressors. This knowledge helps to identify vulnerable stages of the pest and develop appropriate control strategies. For example, mating disruption to target adults, biocontrol agents against multiple life stages, especially eggs and larvae, oils as ovicides, and other pesticides against larvae and other life stages can tackle each stage effectively. Modern tools such as smart traps to monitor pest populations and drones for releasing natural enemies can also help improve the IPM program.
C. Planning and Organization: Since insecticide resistance is a common problem with DBM, rotating pesticides (both biological and synthetic) among different mode of action groups and avoiding repetitive application of the same or a similar pesticide are critical for resistance management. Making appropriate treatment decisions based on infestation levels and the life stage of the pest, regularly monitoring for potential resistance issues, and keeping track of combination and rotation programs that worked well are all a part of effective planning and information management that improve pest control efficacy. If necessary, aggressive area-wide management plans should be developed using one or more control options.
D. Communication: When dealing with an important pest such as DBM, effective communication will help address the knowledge gaps and contribute to effective pest management. Pest control professionals and growers can explore new DBM control options by contacting researchers, attending extension meetings, or reading various articles that can be accessed through internet, university resources, or local governmental agencies. Growers can also exchange information and develop IPM strategies that best suit their situation through a collaborative effort.
Since the field conditions, infestation levels, resistance in DBM populations, and availability and affordability of control options vary, growers should customize their IPM program to suit their local needs.
References
Begna, F. and T. Damtew. 2015. Evaluation of four botanical insecticides against diamondback moth, Plutella xylostella L. (Lepidoptera: Plutellidae) on head cabbage in the central rift valley of Ethiopia. Sky J. Agrl. Res. 4: 97-105. http://www.skyjournals.org/sjar/pdf/2015pdf/Aug/Begna%20and%20Damtaw%20pdf.pdf
Capinera, J. L. 2018. Diamondback moth. Featured Creatures, University of Florida Publication EENY-119. https://entnemdept.ufl.edu/creatures/veg/leaf/diamondback_moth.htm
Dara, S. K. 2019. The new integrated pest management paradigm for the modern age. JIPM 10: 12, 1-9. https://doi.org/10.1093/jipm/pmz010
Dara, S. K. 2020. Mating disruption as an IPM tool in diamondback moth management. UCANR eJournal of Entomology and Biologicals. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=44160
Devi, H. D. and A. R. Tayde. 2017. Comparative efficacy of bio-agents and botanicals on the management of diamondback moth (Plutella xylostella Linn.) on cabbage under Allahabad agroclimatic conditions. Int. J. Curr. Microbiol. App. Sci. 6: 711-716. https://doi.org/10.20546/ijcmas.2017.607.088
Dickson, M. H., A. M. Shelton, S. D. Eigenbrode and M. L. Vamosy. 1990. Selection for resistance to diamondback moth (Plutella xylostella) in cabbage. HortSci. 25: 1643-1646. https://doi.org/10.21273/HORTSCI.25.12.1643
Kumrungsee, N., W. Pluempanupat, O. Koul, and V. Bullangpoti. 2014. Toxicity of essential oil compounds against diamondback moth, Plutella xylostella, and their impact on detoxification enzyme activities. J. Pest Sci. 87: 721-729. https://doi.org/10.1007/s10340-014-0602-6
Ling,B., G.-c. Wang, J. Ya, M.-x. Zhang, and G.-w. Liang. 2008. Antifeedant activity and active ingredients against Plutella xylostella from Momordica charantia leaves. Agrl. Sci. China 7: 1466-1473. https://doi.org/10.1016/S1671-2927(08)60404-6
Lingathurai, S., S. E. Vendan, M. G. Paulraj, and S. Ignacimuthu. 2011. Antifeedant and alrvicidal activities of Acalypha fruticosa Forssk. (Euphorbiaceae) against Plutella xylostella L. (Lepidoptera: Yponomeutidae) larvae. J. King Saud Univ. Sci. 23: 11-16. https://doi.org/10.1016/j.jksus.2010.05.012
Robin, A.H.K., M. R. Hossain, J.-I. Park, H. R. Kim and I.-S. Nou. 2017. Glucosinolate profiles in cabbage genotypes influence the preferential feeding of diamondback moth (Plutella xylostella). Fron. Plant Sci. 8: 1244. https://doi.org/10.3389/fpls.2017.01244
Sarfraz, M., A. B. Keddie, and L. M. Dosdall. 2007. Biological control of the diamondback moth, Plutella xylostella: a review. Biocon. Sci. Tech. 15: 763-789. https://doi.org/10.1080/09583150500136956
Shinoda, T., T. Nagao, M. Nakayama, H. Serizawa, M. Koshioka, H. Okabe, and A. Kawai. 2002. Identification of a triterpenoid saponin from a crucifer, Barbarea vulgaris, as a feeding deterrent to the diamondback moth, Plutella xylostella. J. Chem. Ecol. 28: 587-599. https://doi.org/10.1023/A:1014500330510
https://ucanr.edu/articlefeedback
- Author: Surendra K. Dara
- Author: Roland C. Bocco
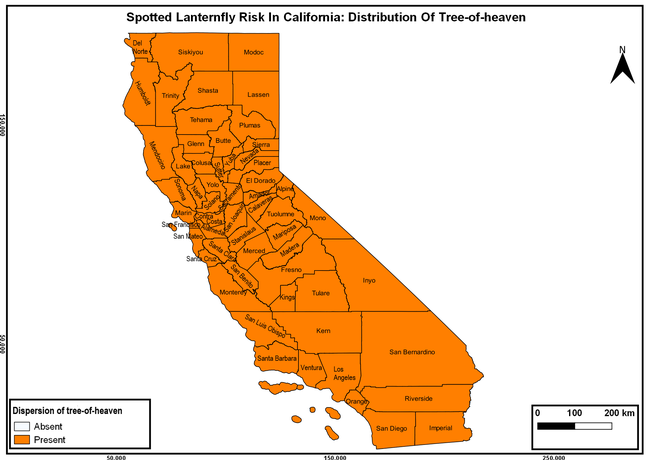
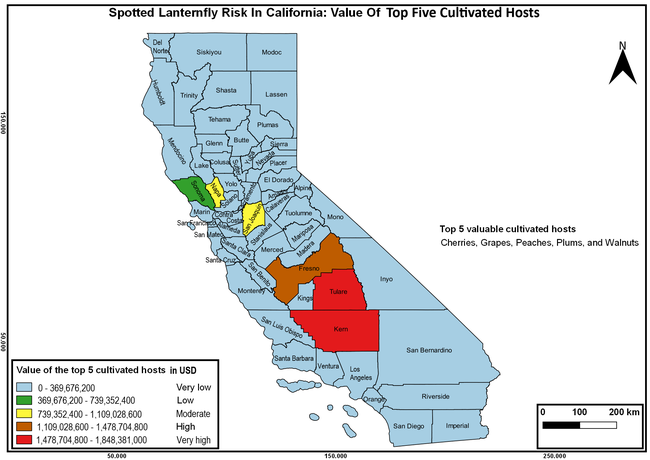
The spotted lanternfly (SLF) [Lycorma delicatula (Hemiptera: Fulgoridae)] is an invasive planthopper, which causes a significant damage to apples, grapes, stone fruit, trees used for timber, and other hosts (Dara et al. 2015). Native to China, SLF was first reported in 2014 in Pennsylvania and has been rapidly spreading in the eastern United States and moving westward. California has 22 cultivated and about 70 wild hosts of SLF and include several high value crops such as apples, cherries, grapes, and plums. The tree-of-heaven, an invasive species, is a favorite host of SLF and is widely distributed in California. SLF is also a nuisance pest with 100s or 1000s of individuals infesting landscape trees and hosts in residential areas. This pest deposits eggs on inanimate objects such as vehicles, furniture, stones, and packages and thus spread to other areas through the movement of these objects. Awareness of the pest and its damage potential, ability of Californians to recognize and report the pest if found, and the knowledge of control practices will help prevent accidental transportation of eggs or other life stages from the infested areas to California and prepare the citizens to take appropriate actions. Outreach efforts have been made in California since 2014 through extension articles, presentations at extension meetings, videos, social media posts, and personal communication (Dara, 2014).
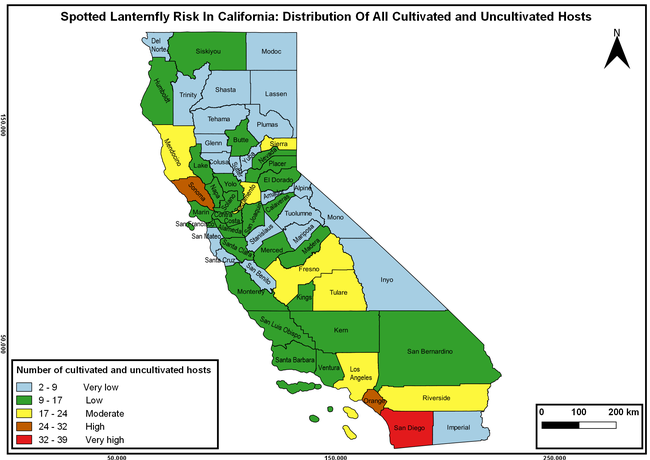
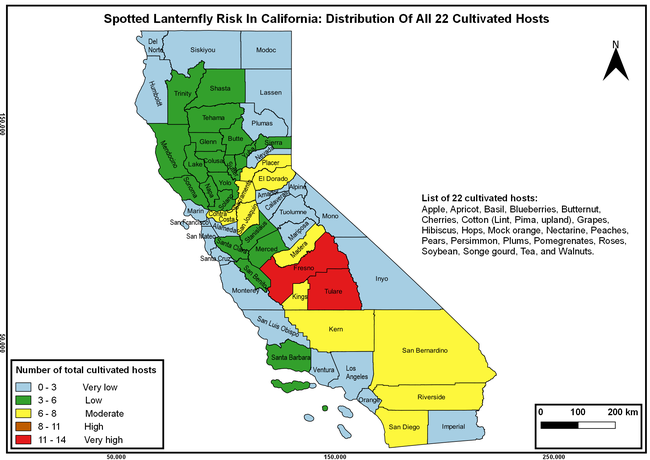
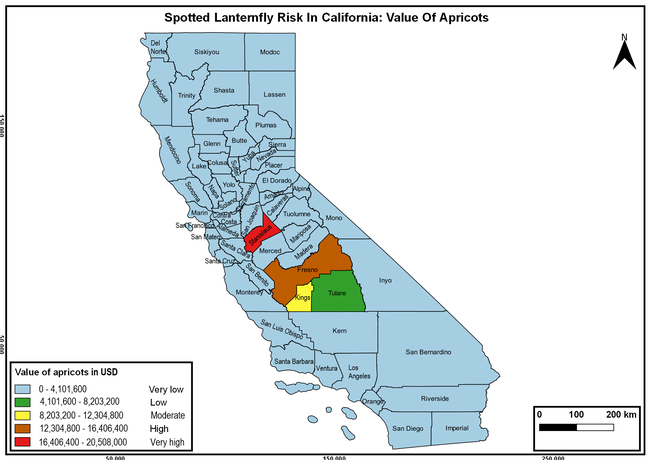
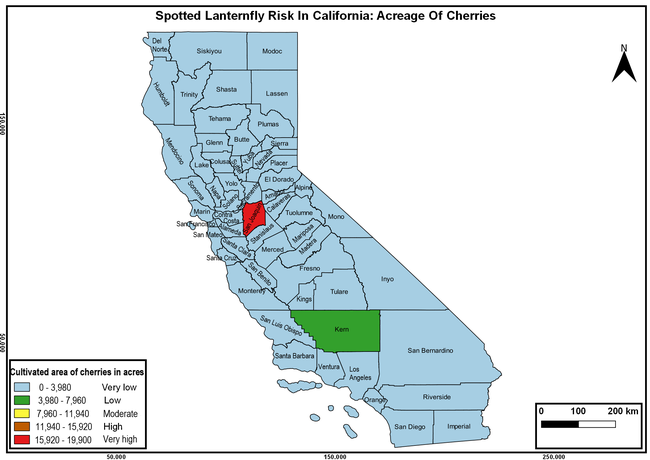
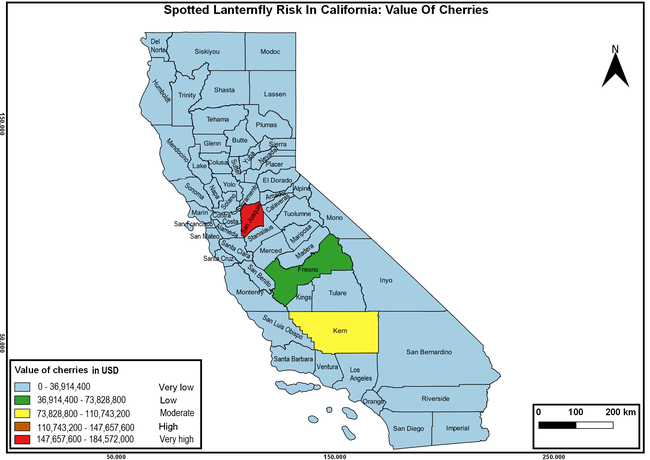
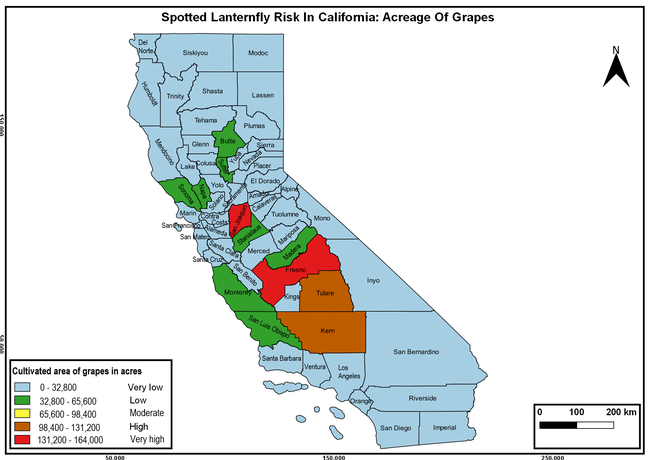
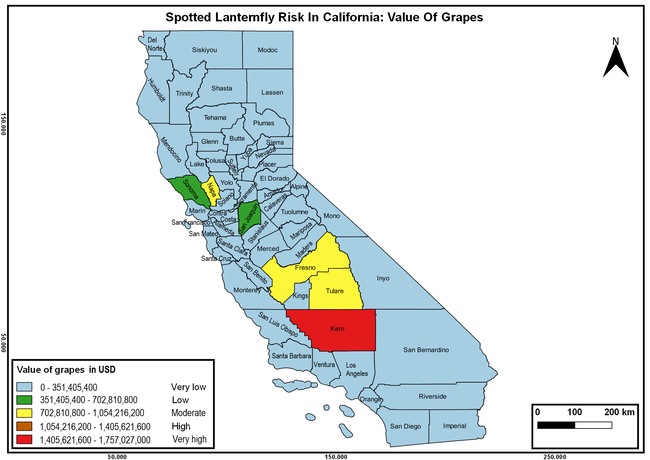
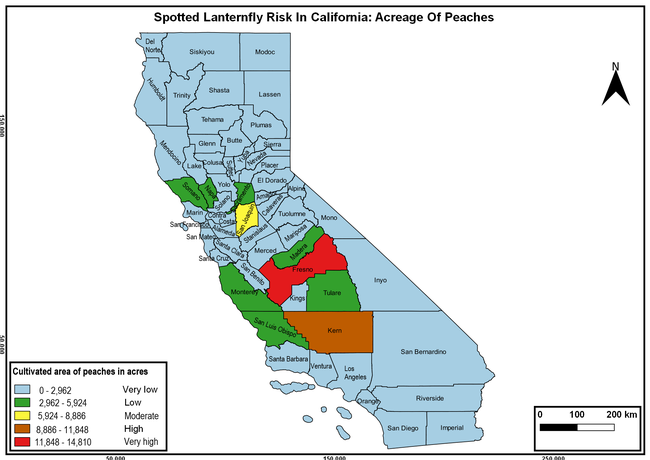
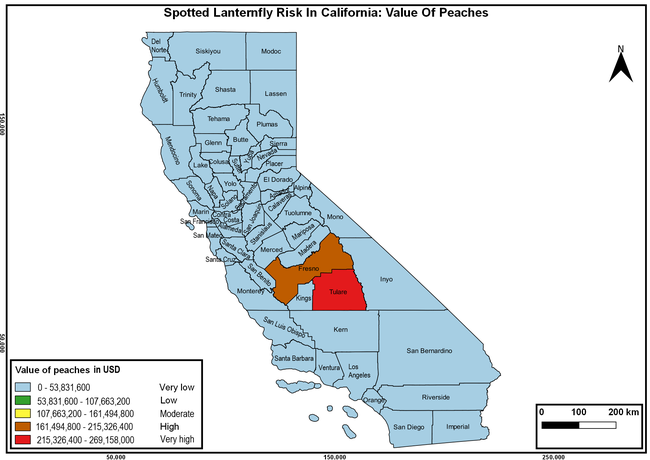
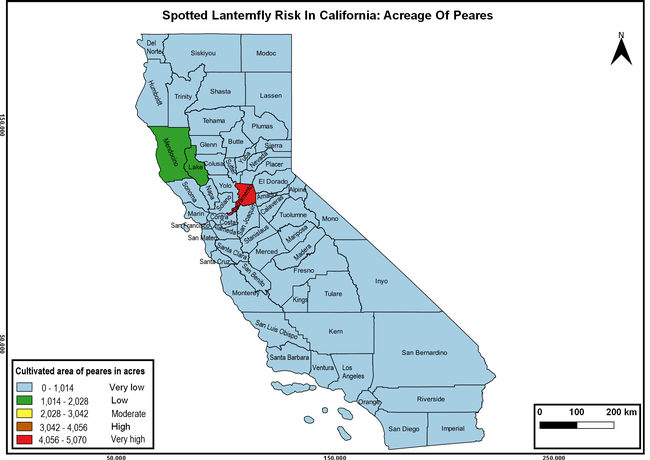
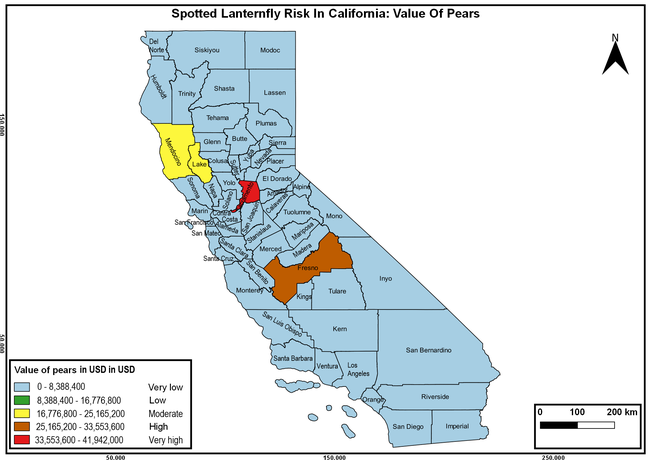
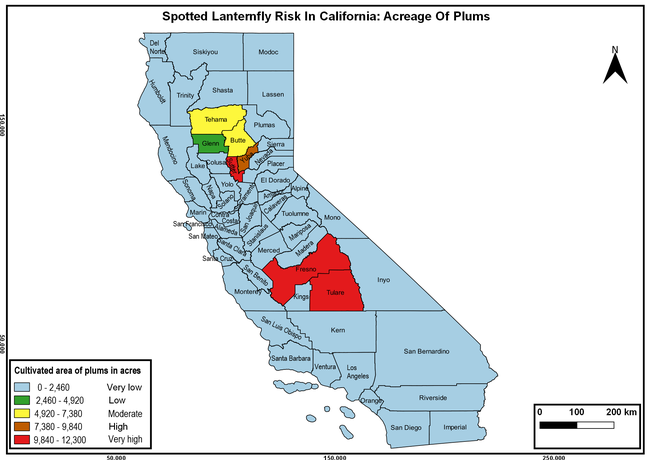
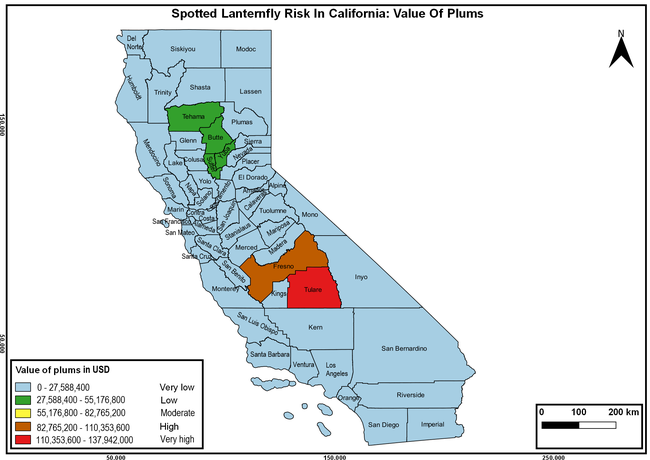
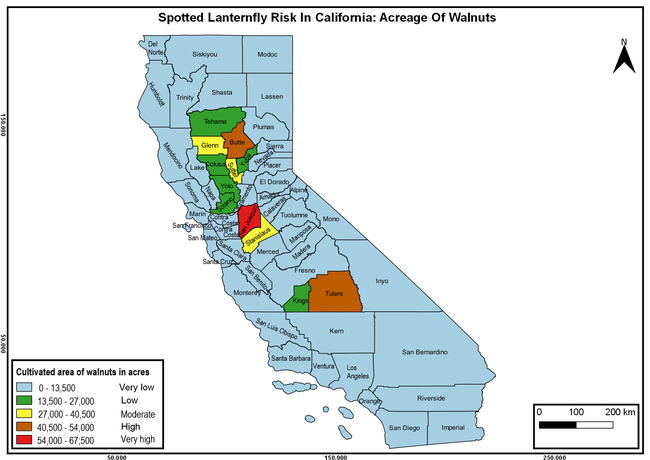
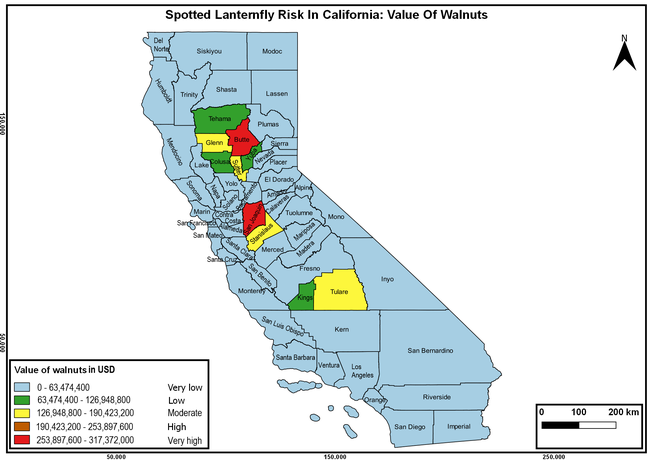
Wakie et al. (2020) modeled the establishment risk of SLF in the United States and around the world and indicated that many coastal regions and the Central Valley of California are among the high-risk areas. Considering the risk to several high-value commodities and the presence of several wild hosts that are distributed all over California, mapping of the risk-prone areas based on the cultivated hosts, their acreage and value in different counties, and the distribution of wild hosts was done to help both growers and other Californians to prepare for potential invasion of SLF.
Methodology
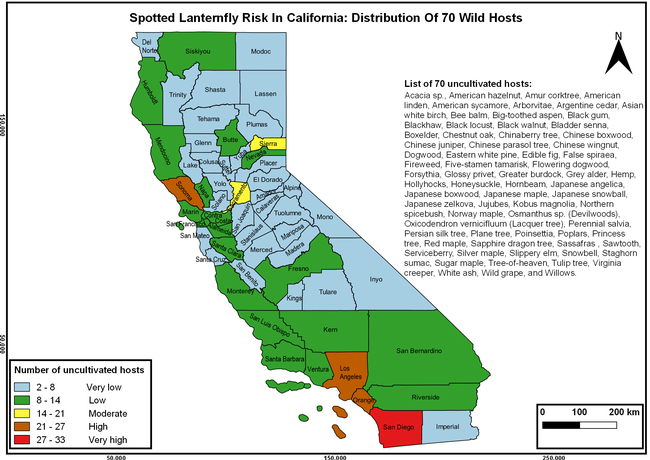
The list of SLF hosts is continuously evolving with host specificity studies in various places. Based on two published resources (Dara et al. 2015; Barringer and Ciafré 2020), 22 cultivated and 70 wild hosts appear to be present in California. Plant species that support some of the feeding life stages or all life stages were included in preparing these lists. The cultivated hosts include apples, apricots, basil, blueberries, butternut, cherries, cotton, grapes, hibiscus, hops, mock orange, nectarine, peaches, pears, persimmon, plums, pomegranates, roses, soybean, sponge gourd, tea, and walnuts; and the wild hosts include Acacia sp., American hazelnut, Amur corktree, American linden, American sycamore, arborvitae, Argentine cedar, Asian white birch, bee balm, big-toothed aspen, black gum, black hawk, black locust, black walnut, Bladder senna, boxelder, chestnut oak, chinaberry tree, Chinese boxwood, Chinese juniper, Chinese parasol tree, Chinese wingnut, devilwoods, dogwood, Eastern white pine, edible fig, false spiraea, fireweed, five-stamen tamarisk, flowering dogwood, Forsythia, Glossy privet, greater burdock, grey alder, hemp, hollyhocks, honeysuckle, hornbeam, Japanese angelica, Japanese boxwood, Japanese maple, Japanese snowball, Japanese zelkova, jujubes, Kobus magnolia, Northern spicebush, Norway maple, lacquer tree, perennial salvia, Persian silk tree, plane tree, Poinsettia, poplars, princess tree, red maple, sapphire dragon tree, sassafras , sawtooth, serviceberry, silver maple, slippery elm, snowbell, staghorn sumac, sugar maple, tree-of-heaven, tulip tree, Virginia creeper, white ash, wild grape, and willows.
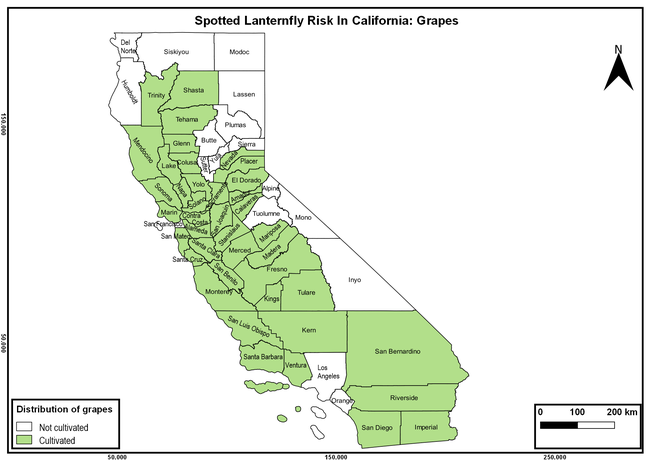
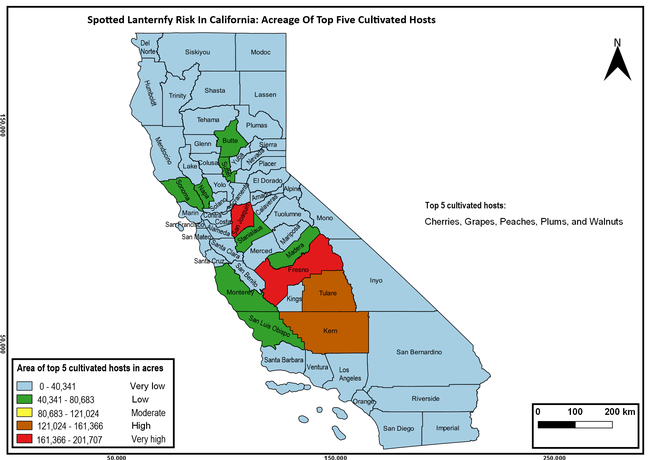
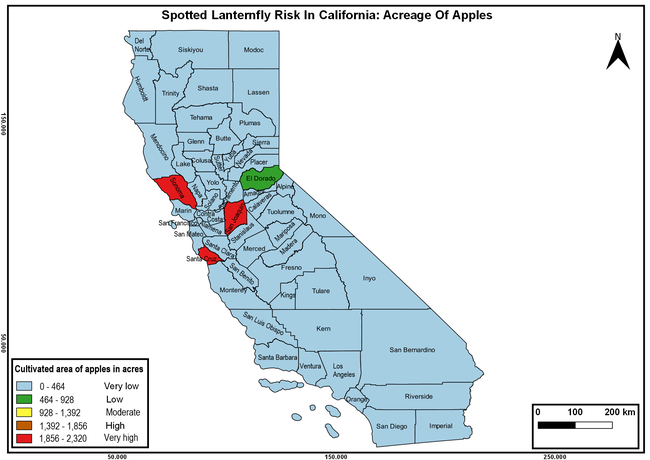
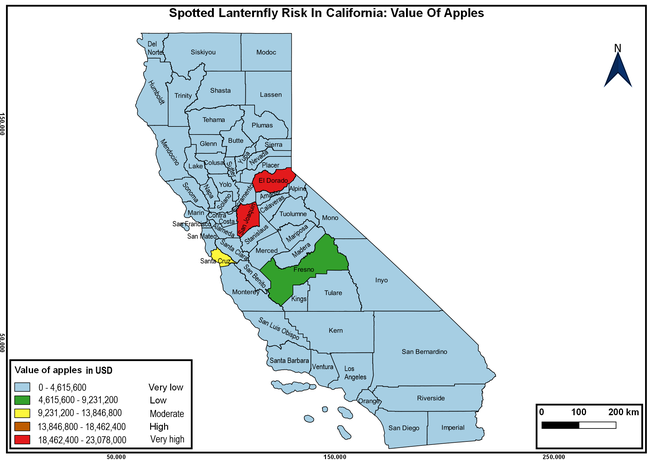
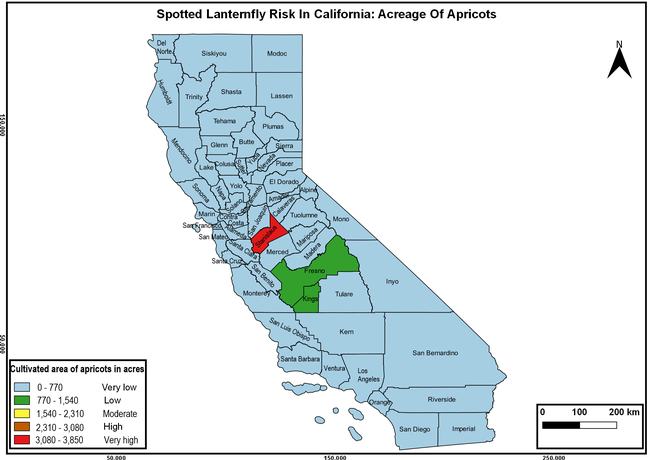
The summary of county crop reports from the California Department of Food and Agriculture (CDFA 2018) was used to determine the value and acreages of the cultivated hosts. To determine the distribution of wild hosts various online resources were used. SLF risk levels were determined as very low, low, moderate, high, and very high for the number of hosts, acreage and value of each cultivated host, and other such parameters within each county. The highest risk value within each parameter was used to determine ‘very high' category and 4/5, 3/5, 2/5, and 1/5 were used for high, moderate, low, and very low categories, respectively. In other words, 0-20% risk was considered very low, 21-40% as low, 41-60% as moderate, 61-80% as high, and 81-100% as very high for each measured parameter. Data were entered into a spreadsheet and maps were generated using QGIS open-source cross-platform geographic information system application.
Risk-prone areas in California
The following maps show areas in California that are prone to SLF risk based on the distribution of cultivated and wild hosts, and the acreage and value of important cultivated crops.
Based on these maps, the entire state of California is at some level of risk. In addition to the commercially produced crops, several backyard or landscape plant species such as roses, grapes, peaches, plums, and others are present throughout the state and can harbor SLF. Such host plants in residential and urban landscapes can serve as SLF sources for commercial crops. The tree-of-heaven is present throughout California and several such uncultivated hosts can serve as sources of undetected infestations. While researchers are working on appropriate biocontrol solutions such as releasing natural enemies, other control options such as synthetic and microbial pesticide applications, sticky traps, removal of egg masses and wild hosts, and other strategies can help manage SLF. In the meantime, Californians will benefit by knowing about this pest and its potential risk to the state. The ability to identify, destroy or capture, and report the pest to county and state departments or University of California Cooperative Extension offices will help prevent or delay SLF invasion and spread in California.
Conclusion
California is at the risk of SLF invasion and spread. Depending on the number of cultivated crops, their acreage, value, and the distribution of wild hosts, the risk level varies in various counties throughout the state. Outreach efforts are helping to alert Californians about SLF and its damage to cultivated crops and nuisance in urban and residential areas.
Additional resources
Refer to https://ucanr.edu/spottedlanternfly for additional information about the pest. If you happen to see this pest in California, please contact your local Agricultural Commissioner, California Department of Food and Agriculture, or UC Cooperative Extension office to report.
Acknowledgments
Thanks to the California Department of Food and Agriculture for funding this study.
References
Barringer, L. and Ciafré, C. M. 2020. Worldwide feeding host plants of spotted lanternfly, with significant additions from North America. Environ. Entomol. 49: 999-1011.
CDFA (California Department of Food and Agriculture). 2018. California County Agricultural Commissioners' Report Crop Year 2016-2017 (https://www.cdfa.ca.gov/statistics/pdfs/2017cropyearcactb00.pdf).
Dara, S. K. 2014. Spotted lanternfly (Lycorma delicatula) is a new invasive pest in the United States. UCANR eJournal Pest News (https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=15861).
Dara, S. K. 2018. An update on the invasive spotted lanternfly, Lycorma delicatula: current distribution, pest detection efforts, and management strategies. UCANR eJournal Pest News (https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=26349).
Dara, S. K., Barringer, L. and Arthurs, S. P. 2015. Lycorma delicatula (Hemiptera: Fulgoridae): a new invasive pest in the United States. J. Integr. Pest Manag. 6: 20.
Wakie, T. T., Nevin, L. G., Yee, W. L. and Lu, Z. 2020. The establishment risk of Lycorma delicatula (Hemiptera: Fulgoridae) in the United States and globally. J. Econ. Entomol. 113: 306-314.